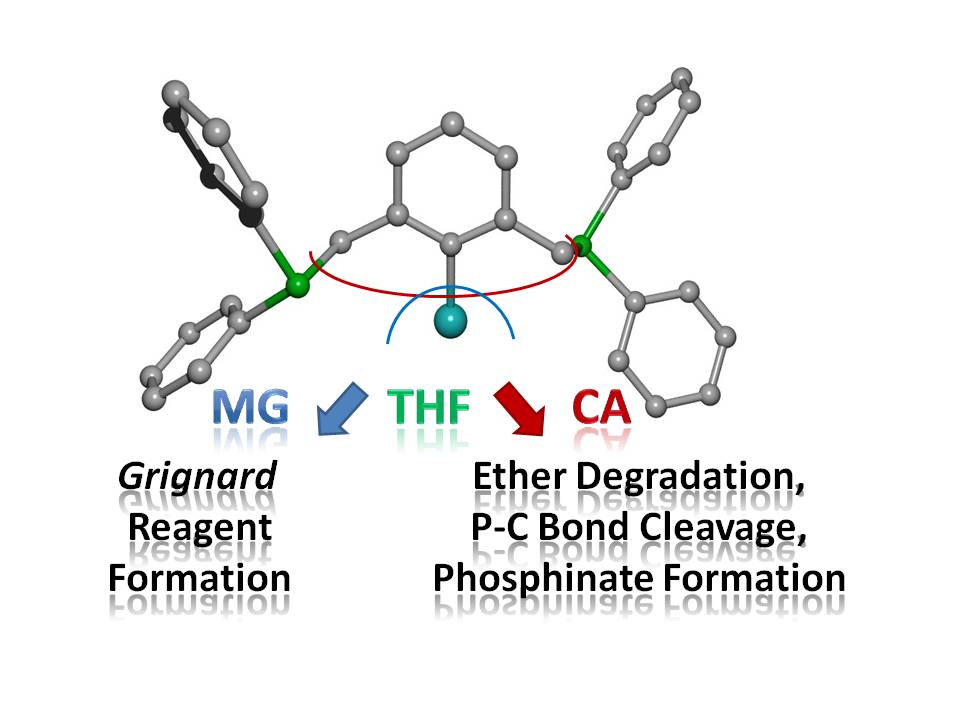
Arylmagnesium and -calcium reagents are easily accessible, however, ether degradation processes limit the storability especially of the calcium-based heavy Grignard reagents. Ortho-bound substituents with phosphanyl donor sites usually block available coordination sites and stabilize such complexes. The reaction of bromo-2,6-bis(diphenylphosphanylmethyl)benzene (1a) with magnesium in tetrahydrofuran yields [Mg{C6H3-2,6-(CH2PPh2)2}2] (2) after recrystallization from 1,2-dimethoxyethane. However, the similarly performed reduction of bromo- (1a) and iodo-2,6-bis(diphenylphosphanylmethyl)benzene (1b) with calcium leads to ether cleavage and subsequent degradation products. α-Deprotonation of THF yields 1,3-bis(diphenylphosphanylmethyl)benzene. Furthermore, the insoluble THF adducts of dimeric calcium diphenylphosphinate halides, [(thf)3Ca(X)(µ-O2PPh2)]2 [X = Br (3a), I (3b)], precipitate verifying ether decomposition and cleavage of P-C bonds. Ether adducts of calcium halides [such as [(dme)2(thf)CaBr2] (4)] form supporting the initial Grignard reaction and a subsequent Schlenk-type dismutation reaction.