Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1.0. Introduction
2.0. Experimental Details
3.0. Models and Discussion
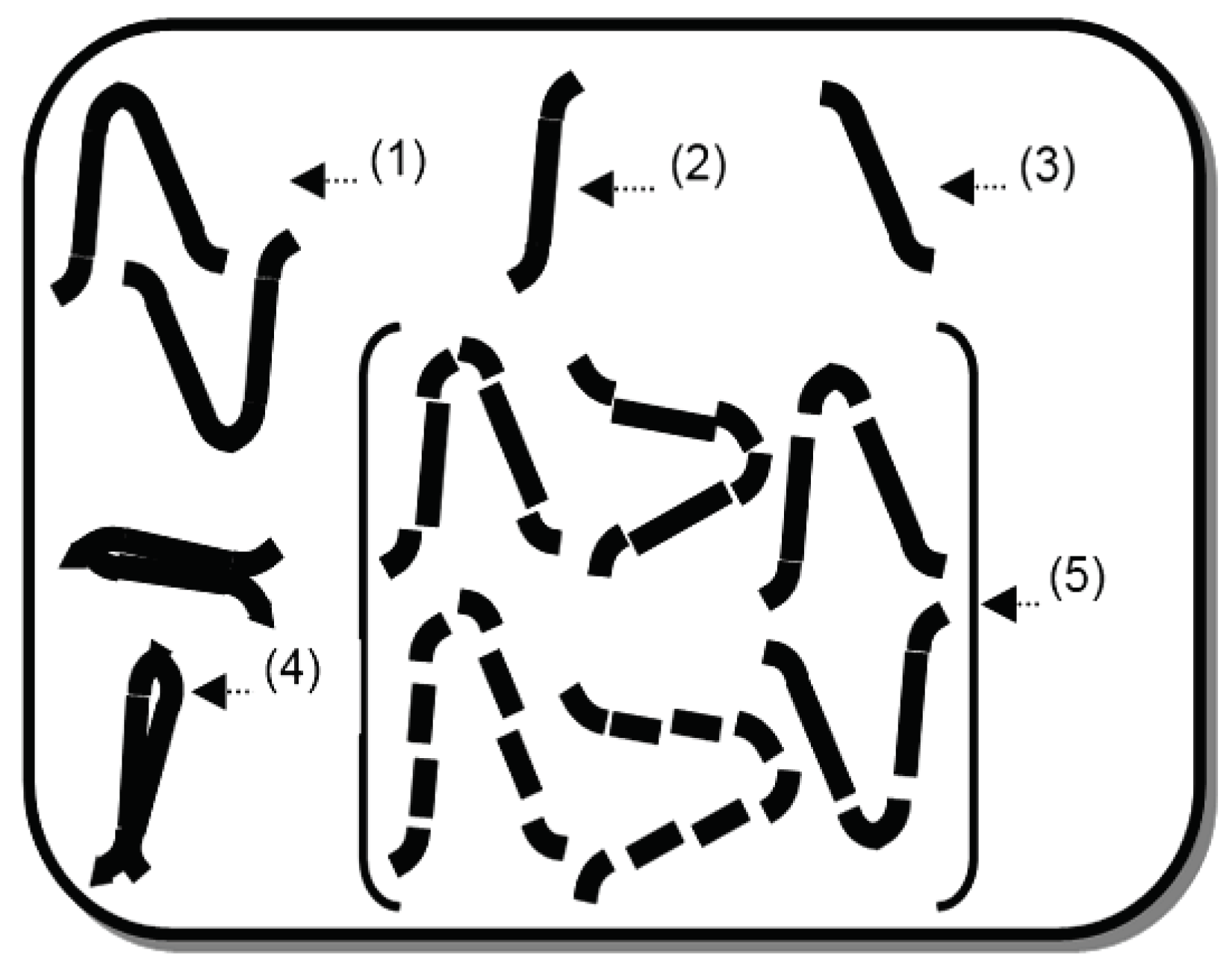
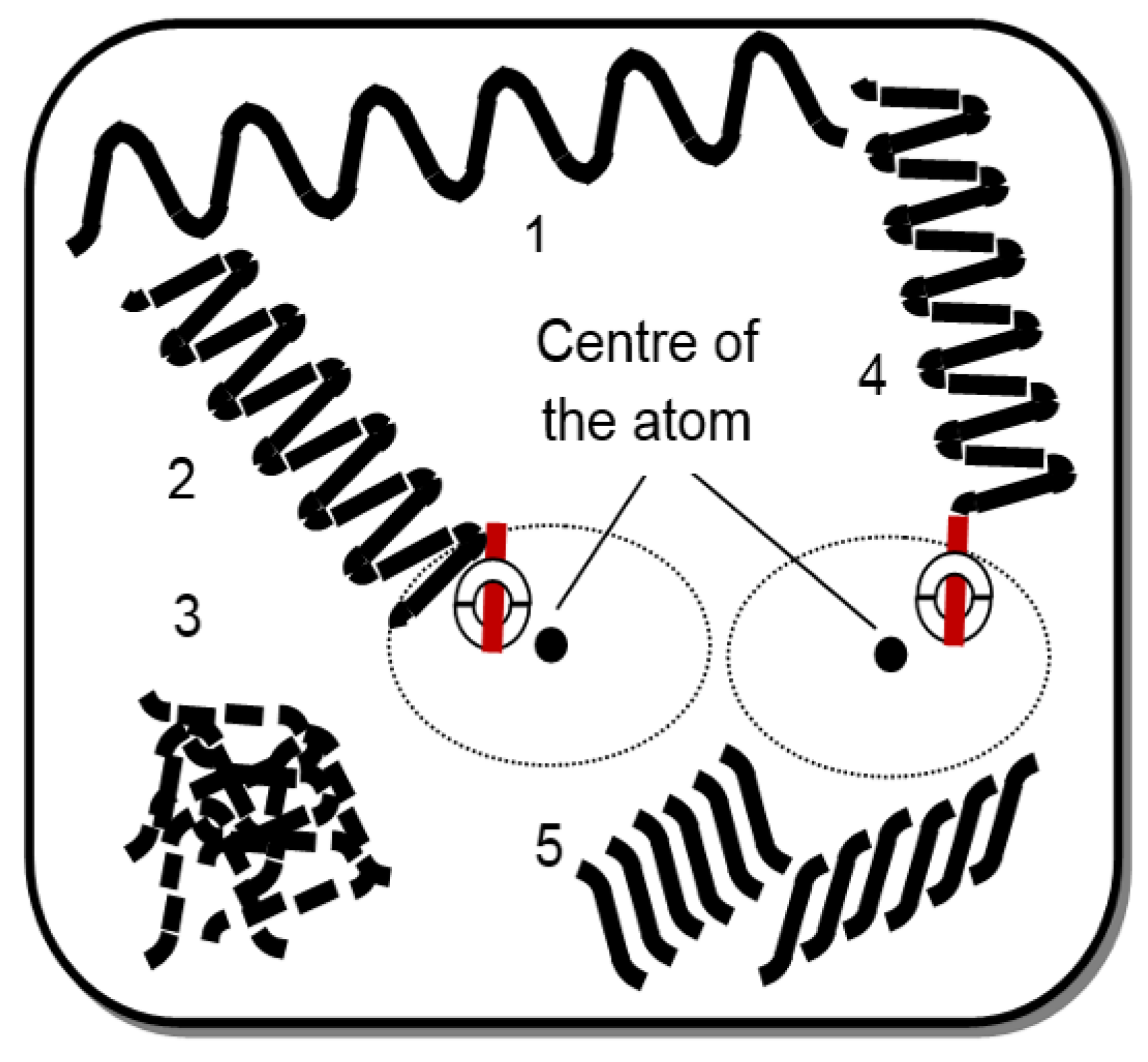
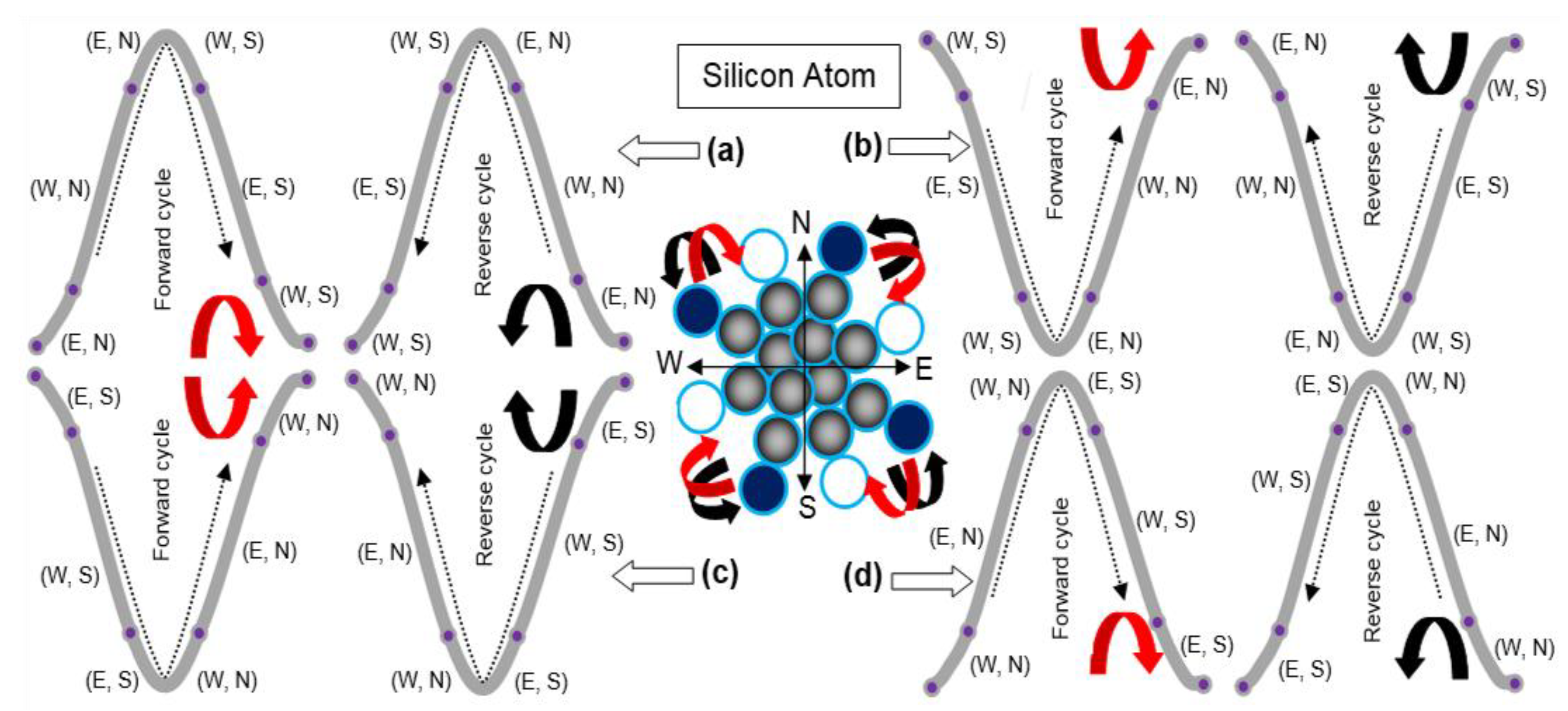
3.1. Heat Energy Phenomenon
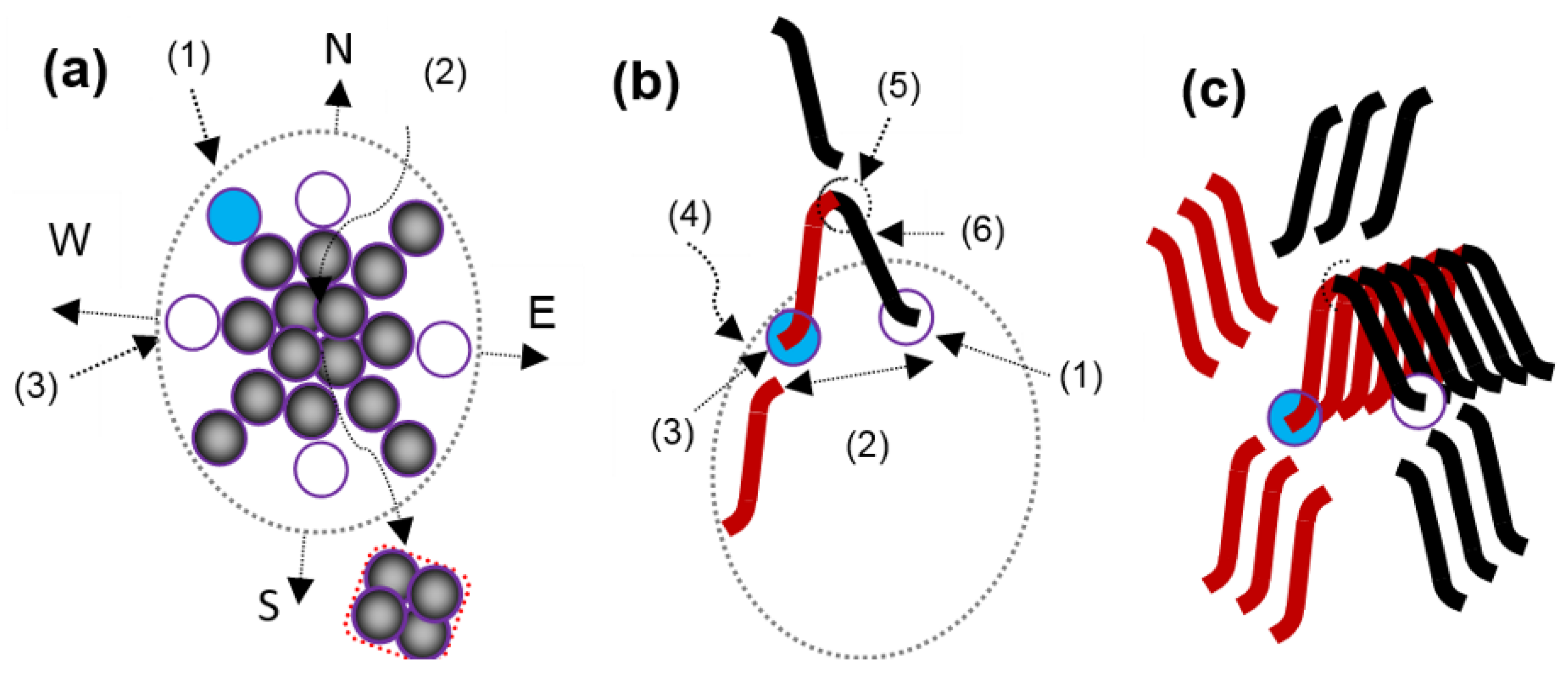
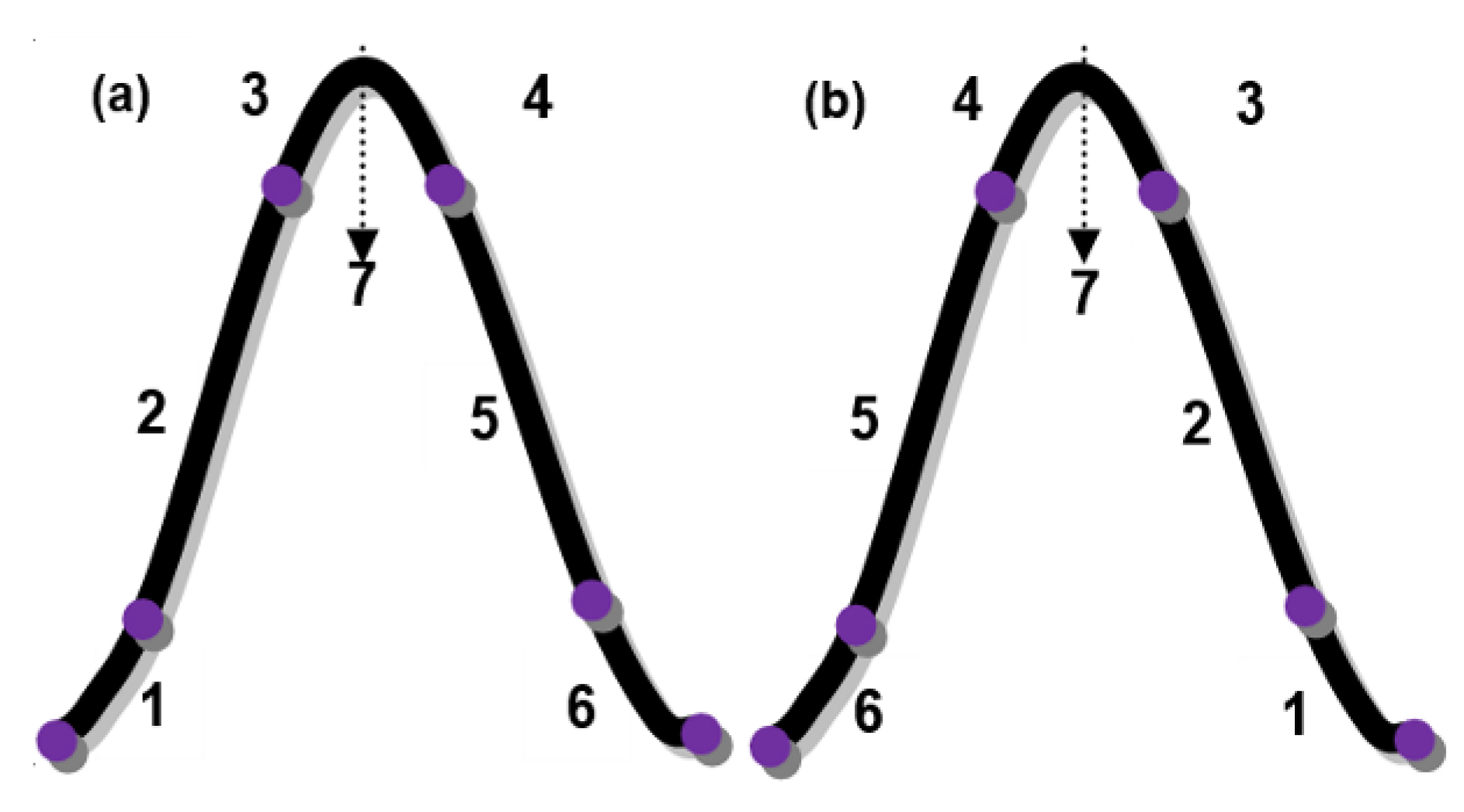
3.2. Photon Energy Phenomenon
3.3. General Discussion
4.0. Conclusion
Data availability statement
Acknowledgments
Conflicts of interest
References
- Bohm, D.; Pines, D. A Collective Description of Electron Interactions. I. Magnetic Interactions. Phys. Rev. B 1951, 82, 625–634. [Google Scholar] [CrossRef]
- D. Pines, D. D. Pines, D. Bohm, A Collective Description of Electron Interactions: II. Collective vs Individual Particle Aspects of the Interactions, Phys. Rev. 85, (1952) 338-353. [CrossRef]
- Ritchie, R.H. Plasma Losses by Fast Electrons in Thin Films. Phys. Rev. B 1957, 106, 874–881. [Google Scholar] [CrossRef]
- Bohm, D.; Pines, D. A Collective Description of Electron Interactions: III. Coulomb Interactions in a Degenerate Electron Gas. Phys. Rev. B 1957, 92, 609–625. [Google Scholar] [CrossRef]
- Low, T.; Chaves, A.; Caldwell, J.D.; Kumar, A.; Fang, N.X.; Avouris, P.; Heinz, T.F.; Guinea, F.; Martin-Moreno, L.; Koppens, F. Polaritons in layered two-dimensional materials. Nat. Mater. 2017, 16, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J. On the Transformation of light into Heat in Solids. I. Phys. Rev. B 1931, 37, 17–44. [Google Scholar] [CrossRef]
- Couto, O.D.D.; Puebla, J.; Chekhovich, E.A.; Luxmoore, I.J.; Elliott, C.J.; Babazadeh, N.; Skolnick, M.S.; Tartakovskii, A.I.; Krysa, A.B. Charge control in InP/(Ga,In)P single quantum dots embedded in Schottky diodes. Phys. Rev. B 2011, 84, 125301. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Whetten, R.L.; Khoury, J.T.; Alvarez, M.M.; Murthy, S.; Vezmar, I.; Wang, Z.L.; Stephens, P.W.; Cleveland, C.L.; Luedtke, W.D.; Landman, U. Nanocrystal gold molecules. Adv. Mater. 1996, 8, 428–433. [Google Scholar] [CrossRef]
- Brust, M.; Kiely, C.J. Some recent advances in nanostructure preparation from gold and silver particles: a short topical review. Colloids Surfaces A: Physicochem. Eng. Asp. 2002, 202, 175–186. [Google Scholar] [CrossRef]
- Glotzer, S.C.; Solomon, M.J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 2007, 6, 557–562. [Google Scholar] [CrossRef]
- S. Link, M. A. S. Link, M. A. El-Sayed, Shape and size dependence of radiative, nonradiative and photothermal properties of gold nanocrystals, Int. Rev. Phys. Chem. 19, (2000) 409- 453. [CrossRef]
- Shaw, C.P.; Fernig, D.G.; Lévy, R. Gold nanoparticles as advanced building blocks for nanoscale self-assembled systems. J. Mater. Chem. 2011, 21, 12181–12187. [Google Scholar] [CrossRef]
- Negishi, Y.; Nakazaki, T.; Malola, S.; Takano, S.; Niihori, Y.; Kurashige, W.; Yamazoe, S.; Tsukuda, T.; Häkkinen, H. A Critical Size for Emergence of Nonbulk Electronic and Geometric Structures in Dodecanethiolate-Protected Au Clusters. J. Am. Chem. Soc. 2015, 137, 1206–1212. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N. Forces driving amalgamation of nanoparticles and particles in solution. Forces Mech. 2022, 7. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N. Development of gold tiny particles and particles in different sizes at varying precursor concentration. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015006. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N. Controlling morphology-structure of gold tiny particles, nanoparticles and particles at different pulse rates and pulse polarity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025015. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N. Formation of tiny particles and their extended shapes: origin of physics and chemistry of materials. Appl. Nanosci. 2019, 9, 1367–1382. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-. .-N.; Yeh, C.-J. Tapping Opportunity of Tiny-Shaped Particles and Role of Precursor in Developing Shaped Particles. Nano 2018, 13. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I. Phase transitions and critical phenomena of tiny grains carbon films synthesized in microwave-based vapor deposition system. Surf. Interface Anal. 2019, 51, 389–399. [Google Scholar] [CrossRef]
- Ali, M.; Ürgen, M. Switching dynamics of morphology-structure in chemically deposited carbon films – A new insight. Carbon 2017, 122, 653–663. [Google Scholar] [CrossRef]
- M. Ali, Tiny-Shaped Particles Developing a Mono-Layer Shape Dealing Localized Gravity and Levity at the Solution Surface, (2024). http://arxiv.org/abs/1609.08047v34. [CrossRef]
- M. Ali, Atoms of None of the Elements Ionize While Atoms of Inert Behavior Split by Photonic Current, (2024). http://arxiv.org/abs/1611.05392v33. [CrossRef]
- M. Ali, Atoms in Gaseous and Solid States and Their Energy and Force Relationships under Transitional Behaviors, (2023). [CrossRef]
- M. Ali, Structural Evolutions in Atoms of the Elements Executing Confined Interstate Electron Dynamics, (2023). http://arxiv.org/abs/1611.01255v32. [CrossRef]
- M. Ali, Atomic Structure and Binding of Carbon Atoms, (2023). https://www.preprints.org/manuscript/201801.0036/v17. [CrossRef]
- Ali, M.; Lin, I.-N. Gold Nanostructures and Microstructures with Tunable Aspect Ratios for High-Speed Uni- and Multidirectional Photonic Applications. ACS Appl. Nano Mater. 2020, 3, 9410–9424. [Google Scholar] [CrossRef]
- J. Zhao, L. J. Zhao, L. Yang, Structure Evolutions and Metallic Transitions in In2Se3 Under High Pressure, J. Phys. Chem. C 118, (2014) 5445-5452. [CrossRef]
- Manoharan, V.N. Colloidal matter: Packing, geometry, and entropy. Science 2015, 349, 1253751. [Google Scholar] [CrossRef]
- Park, J.; Elmlund, H.; Ercius, P.; Yuk, J.M.; Limmer, D.T.; Chen, Q.; Kim, K.; Han, S.H.; Weitz, D.A.; Zettl, A.; et al. 3D structure of individual nanocrystals in solution by electron microscopy. Science 2015, 349, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, D.; Panciera, F.; Tersoff, J.; Reuter, M.C.; Lehmann, S.; Hofmann, S.; Dick, K.A.; Ross, F.M. Interface dynamics and crystal phase switching in GaAs nanowires. Nature 2016, 531, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tuma, T.; Pantazi, A.; Le Gallo, M.; Sebastian, A.; Eleftheriou, E. Stochastic phase-change neurons. Nat. Nanotechnol. 2016, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rensberg, J.; Zhang, S.; Zhou, Y.; McLeod, A.S.; Schwarz, C.; Goldflam, M.; Liu, M.; Kerbusch, J.; Nawrodt, R.; Ramanathan, S.; et al. Active Optical Metasurfaces Based on Defect-Engineered Phase-Transition Materials. Nano Lett. 2016, 16, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Cardone, G.; Restrepo, D.; Zavattieri, P.D.; Baker, T.S.; Tezcan, F.A. Self-assembly of coherently dynamic, auxetic, two-dimensional protein crystals. Nature 2016, 533, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Evers, C.H.J.; Luiken, J.A.; Bolhuis, P.G.; Kegel, W.K. Self-assembly of microcapsules via colloidal bond hybridization and anisotropy. Nature 2016, 534, 364–368. [Google Scholar] [CrossRef]
- Epstein, I.R.; Xu, B. Reaction–diffusion processes at the nano- and microscales. Nat. Nanotechnol. 2016, 11, 312–319. [Google Scholar] [CrossRef]
- Azizi, A.; Wang, Y.; Lin, Z.; Wang, K.; Elias, A.L.; Terrones, M.; Crespi, V.H.; Alem, N. Spontaneous Formation of Atomically Thin Stripes in Transition Metal Dichalcogenide Monolayers. Nano Lett. 2016, 16, 6982–6987. [Google Scholar] [CrossRef]
- Ali, M. Etching of photon energy into binding energy in depositing carbon films at different chamber pressures. J. Mater. Sci. Mater. Electron. 2023, 34, 1–13. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N.; Yeh, C.-J. Predictor Packing in Developing Unprecedented Shaped Colloidal Particles. Nano 2018, 13. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Hubbard, W.A.; White, E.R.; Dhall, R.; Cronin, S.B.; Aloni, S.; Regan, B.C. Nanoscale temperature mapping in operating microelectronic devices. Science 2015, 347, 629–632. [Google Scholar] [CrossRef]
- Ye, L.; Hou, D.; Zheng, X.; Yan, Y.; Di Ventra, M. Local temperatures of strongly-correlated quantum dots out of equilibrium. Phys. Rev. B 2015, 91, 205106. [Google Scholar] [CrossRef]
- Menges, F.; Mensch, P.; Schmid, H.; Riel, H.; Stemmer, A.; Gotsmann, B. Temperature mapping of operating nanoscale devices by scanning probe thermometry. Nat. Commun. 2016, 7, 10874. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Foster, A.S.; Björkman, T.; Nowakowska, S.; Björk, J.; Canova, F.F.; Gade, L.H.; Jung, T.A.; Meyer, E. Van der Waals interactions and the limits of isolated atom models at interfaces. Nat. Commun. 2016, 7, 11559. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, A.; Ferri, N.; DiStasio, R.A.; Tkatchenko, A. Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science 2016, 351, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
Bibliographic detail:

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




