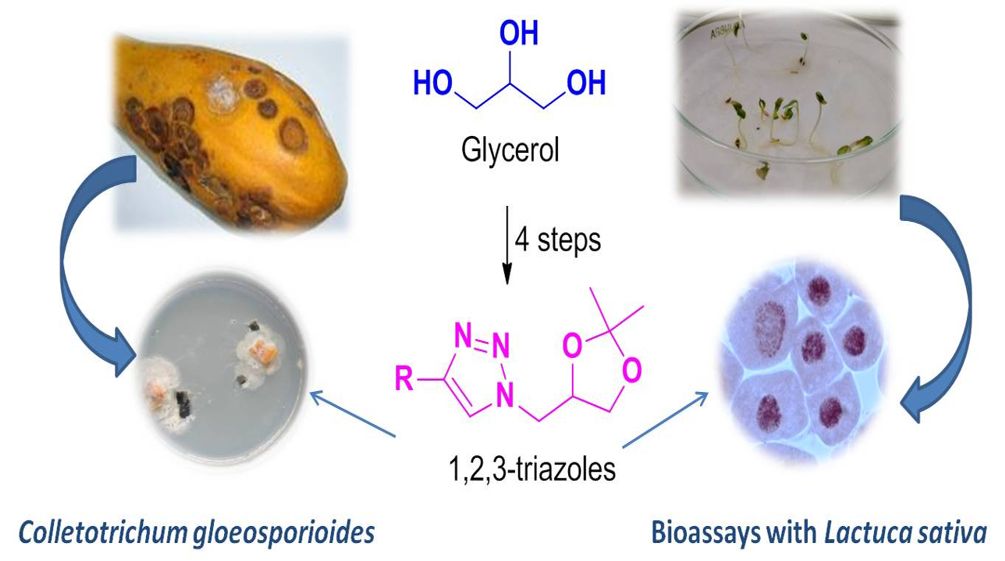
Glycerol is a subproduct of biodiesel production and represents an important problem when generated in large scale. Alternatives that can utilize this unrefined byproduct is of potential interest. It is herein described the synthesis of a series of 1,2,3-triazoles using glycerol as starting material. The key step involved in the preparation of triazolic derivatives corresponded to the Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC), also known as click reaction, between 4-(azidomethyl)-2,2-dimethyl-1,3-dioxolane (3) and different terminal alkynes. The eight prepared derivatives were evaluated with regard to their fungicide, phytotoxic and cytotoxic activities. The fungicide activity was assessed in vitro against Colletotrichum gloeosporioides, the causing agent of papaya anthracnose. It was found that the compounds 1-(1-((2,2-dimethyl-1,3-dioxolan-4-yl)methyl)-1H-1,2,3-triazol-4-yl)cyclohexanol (4g) and 2-(1-((2,2-dimethyl-1,3-dioxolan-4-yl)methyl)-1H-1,2,3-triazol-4-yl)propan-2-ol (4h) demonstrated high efficiency on controlling C. gloeosporioides when compared to the commercial fungicide tebuconazole. The triazoles did not present any phytotoxic effect when evaluated against Lactuca sativa. However, five derivatives were mitodepressive, inducing cell death detected by the presence of condensed nuclei and acted as aneugenic agents in the cell cycle of L. sativa. It is believed that glycerol derivatives bearing 1,2,3-triazole functionalities may represent a scaffold to be explored toward the development of new agents to control C. gloeosporioides.