1.0. Introduction
New strategies are required to process and synthesize carbon-based materials in selective sizes. Characterizations and analyses of carbon-based materials can explore a new science at both primary and applied levels. A force exerted at the electron level should also explain the role of energy at the electron level [
1,
2,
3].
In the structural formation of those carbon atoms where partially conserved energy involves at the electron level, a partially conserved force should also engage at the electron level. It should be the case in the structural formation of graphite, nanotube, and fullerene state atoms.
In the structural formation of those carbon atoms where non-conserved energy involves at the electron level, a non-conserved force should also engage at the electron level. It should be possible in the structural formation of diamond, lonsdaleite, and graphene state atoms. In the carbon atoms where neither energy involves nor force engages at the electron levels, force energy, a chemical in nature, should together contribute at the atomic level to form a structure.
The involvement of the energy at the electron level directs the force to engage at the electron level. As a result, atoms bind to form nano or micro-sized grains. The outer ring electrons of carbon atoms in any state do not entertain a conservative force because of having a very close distance to the center. The nature of the involved energy should depend on the allotrope of a carbon atom. In the literature, several studies discussed the allotropic forms of carbon.
When the conservative forces exert on the electron in a silicon atom, an uninterrupted execution of its dynamics generates a photon of unending length [
2]. It indicates that the built-in interstate gap of electron dynamics in the carbon atom differs from silicon. Both carbon and silicon atoms possess equal numbers of filled and unfilled states in the outer ring. But from the center, the outer ring electrons of a carbon atom have different distances from the outer ring electrons of a silicon atom.
Gaseous and solid atoms deal with the transitions while undertaking the liquid states, where the electrons remain within their occupied energy knots [
3].
The atoms which execute confined interstate electron dynamics evolve structure instead to develop it [
4]. Atoms belonging to all elements do not ionize [
5]. Carbon films in tiny-sized grains got deposited due to the synthetic protocol [
6]. Carbon films of different morphology of grains got deposited at varying process conditions [
7].
At different chamber pressures, the deposition of the carbon films shows different morphology and structure [
8]. At different inter-wire distances, a carbon film was deposited in diamond and graphite phases [
9]. Thus, the electron transfer mechanism is responsible for changing an atom’s chemical nature regardless of whether it belongs to the same element.
The force entering from the north pole and leaving the ground surface for the south pole behaves differently than the force exerted at the ground surface [
10]. A recent study shows the transformation of graphene film into a diamond-like carbon film, where the elastic deformations and chemical natures were changed [
11]. Wu
et al. [
12] reviewed the developments in Raman spectroscopy of graphene materials.
Carbon nanofibers were grown without a catalyst using a vapor deposition method [
13]. Different applications related to graphene hybrids were reviewed [
14]. Nitrogen-incorporated carbon dots merged to modify a glassy carbon electrode [
15]. A novel energy dissipation system was investigated by gathering the features of both carbon nanotubes and fullerenes [
16]. Different carbon allotropes, in comparison, were studied for dehydrogenation of temperature [
17]. Precise positioning of the vacancies in a diamond crystal was studied [
18]. Liu
et al. [
19] presented an efficient strategy to fabricate the graphite-graphene Janus architecture. Some parameters under optimized conditions of the process chosen to deposit the diamond [
20].
Cheng and Zong [
21] observed a structural evolution of damaged carbon atoms for a deeper surface layer. Maruyama and Okada [
22] investigated the electronic and magnetic structures of a two-dimensional network of carbon atoms. Narjabadifam
et al. [
23] studied carbon nanocones’ elastic and failure properties through molecular dynamics simulation. Levitated nanodiamonds burn in the air because of the amorphous carbon on their surfaces [
24]. Uncertainty in the temperature measurement of levitated nanodiamonds was removed [
25].
Heat treatment improves the mechanical properties of carbon films deposited by magnetron sputtering [
26]. A carbon nanotube film improves electrode stability [
27]. Carbon films are deposited in a pulse-based CVD system to improve tribological properties [
28]. By establishing covalent bonds between the substrate and carbon film, the electronic states of carbon-based materials are controlled [
29]. The relation of different parameters with the formation of carbon films was studied [
30]. High negative bias voltages reduce the hydrogen content in depositing carbon film [
31].
The carbon films deposited with an enhanced thickness are not beneficial for all purposes [
32]. The structure and electrochemical properties of carbon films deposited by the electron cyclotron resonance sputtering method have been discussed elsewhere [
33]. A graphitic phase of deposited carbon films reduces the friction coefficient in the vacuum medium [
34]. Carbon films have lowered hydrogen content deposited by tuning the ratio of the graphitic and diamond phases [
35].
The hardness of a single-walled carbon nanotube has been discussed [
36]. Carbon nanotube thin film deposited by the floating catalyst CVD technique offers potential application as a thin film transistor [
37]. Carbon nanotube films discussed their high thermal conductivity [
38]. A recent study suggests the photochemical conversion of the graphitic phase into the diamond phase [
39]. In the deposition of diamond-like carbon films, the sp
2 carbon or graphitic phase increased by introducing titanium [
40]. A structural and electrical relationship establish between amorphous carbon and graphene in deposited carbon films by hot filament CVD [
41]. A recent study investigates porous carbon films for efficient electromagnetic applications [
42]. Vertical mesoporous carbon films were investigated by studying their electrochemical sensors application [
43]. Thermal stability and diffusion characteristics of ultrathin amorphous carbon films have been investigated [
44].
How do the different allotropes of carbon form? How do the same-state carbon atoms bind in the formation of their structures? This study explores fundamental and applied science about carbon atoms. If one looks at the published studies of carbon, it is possible to predict the models related to different allotropes of carbon.
The current study discusses the formation of different states of carbon atoms along with their binding mechanisms. The present work also discusses the formation mechanism of the glassy carbon structure. The current study identifies the kind of energy involved and the nature of forces engaged in the binding of same-state carbon atoms. This study also sketches the Mohs hardness of carbon materials.
2.0. Experimental details
Many published studies on carbon films and other carbon-based materials describe their experimental details. To deposit carbon films is extensively discussed in the literature. There are several techniques in the literature showing the deposition.
The focus of the current study is to explore the underlying science of the different states of a carbon element. A binding mechanism in identical-state carbon atoms is also a subject of this study. The discussed science here is the central subject of every study considering the depositions of carbon materials in any form.
A carbon element not only contributes to describing the experimental detail of the dynamics of the environment but also describes the experimental detail of many other phenomena. The current study also charts the Mohs hardness of different carbon materials under a new insight.
3.0. Atomic Structure Models and Discussion
3.1. Carbon lattice and different carbon states
Presently, the atomic structures of different carbon states do not present a clear picture despite the fact each carbon state keeps a name. The construction mechanism in the carbon lattice is also yet awaited.
Different-state carbon atoms rely on the same number of electrons. A carbon atom has a fixed number of filled and unfilled states in any state. Changing the position of the electrons gives birth to the new chemistry of that atom. However, a separate study discussed the atomic structure of different elements and their transitional behaviors [
3].
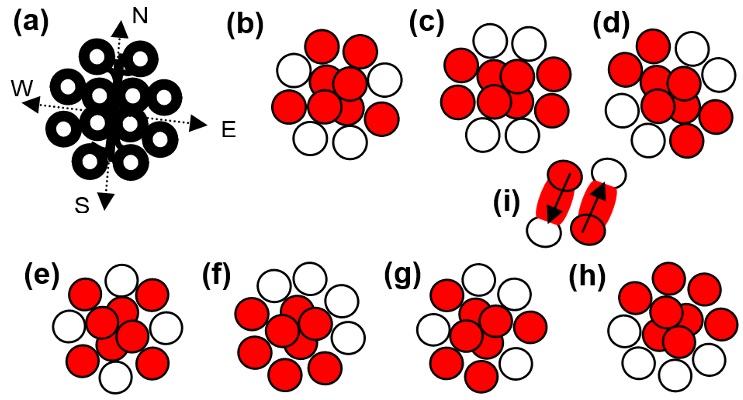
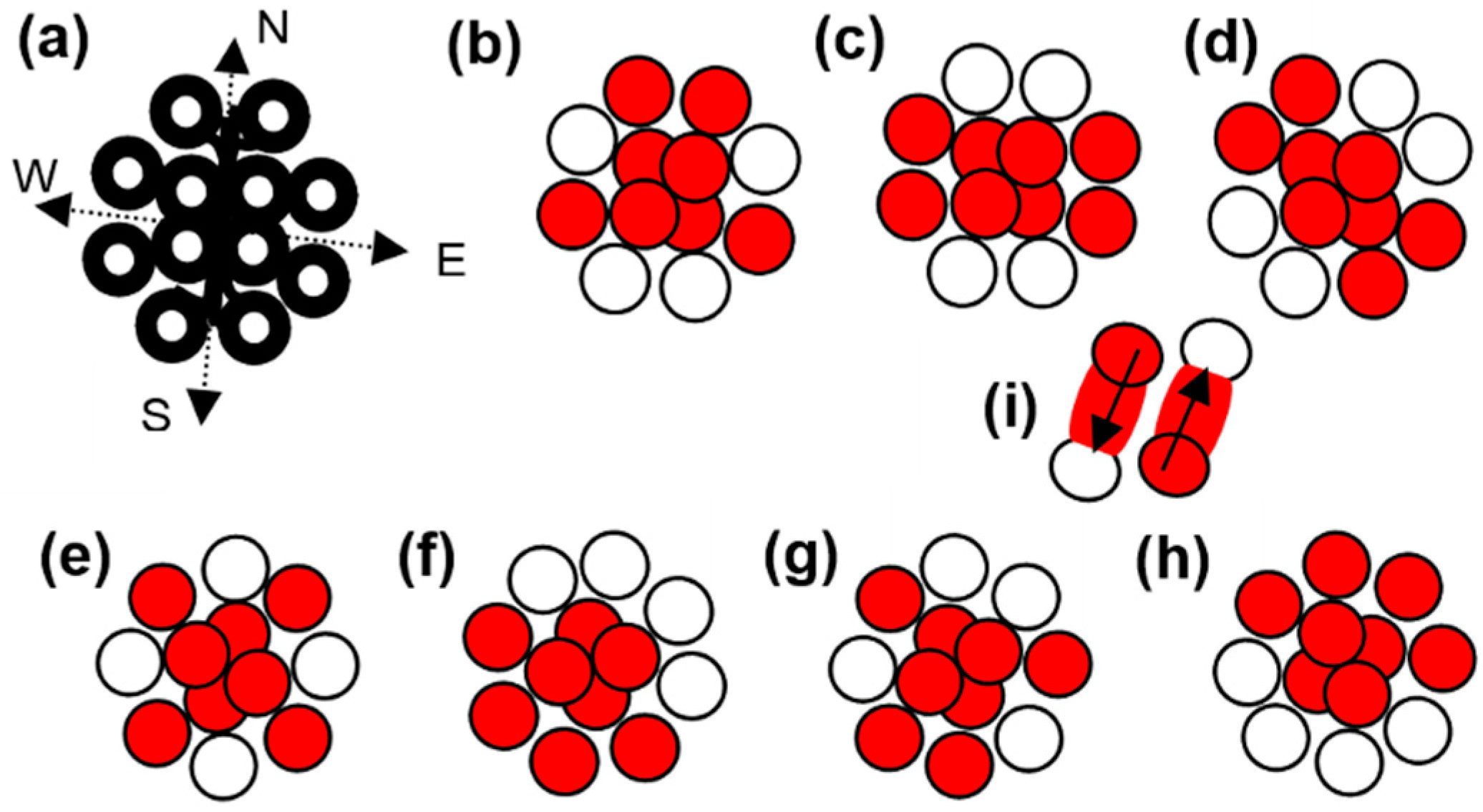
When overt photons intercrossed to form the twelve energy knots, they shape the carbon lattice. In intercrossing, overt photons keep the centers of their lengths at a common point.
Figure 1 (a) shows the lattice of a carbon atom. Two energy knots from each side of the center remained compressed from the neighboring states.
The lengths of the overt photons are so that their schedule crossing shapes the filled and unfilled states required to construct the energy-knot-net of a carbon atom. Two pairs of overt photons, which have characteristics of photonic current, intercross along the east and west sides. Two pairs of photons, which have characteristics of photonic current, intercross along the north and south lines. All the intercrossed overt photons keep the positions of their mid-lengths at the same point. The lattice of a carbon atom is related to the energy-knot-net, as shown in
Figure 1 (a). The overt photons preserve their force by wrapping the energy [
2].
The outer ring of the carbon atom has four filled and four unfilled states. This order of the states provides the option to originate six different states of the carbon atom in addition to the gaseous state and glassy carbon.
Figure 1 shows a gaseous state in (b), a graphite state in (c), a nanotube state in (d), a fullerene state in (e), a diamond state in (f), a lonsdaleite state in (g), and a graphene state in (h). In different states of a carbon atom, four electrons at the center form the zeroth ring. The zeroth ring is related to the helium atom [
3].
Figure 1 symbolically shows the electrons and energy knots in different states of carbon atoms.
3.2. Electron Transfer Mechanism
To convert one state of the carbon atom to another, the etching of carbon precursors, carbon atoms, or methyl radicals remain the main topic of study previously. In a new idea, it is not the case. As discussed [
8], photon energy is etched into binding energy under the application of atomic hydrogen.
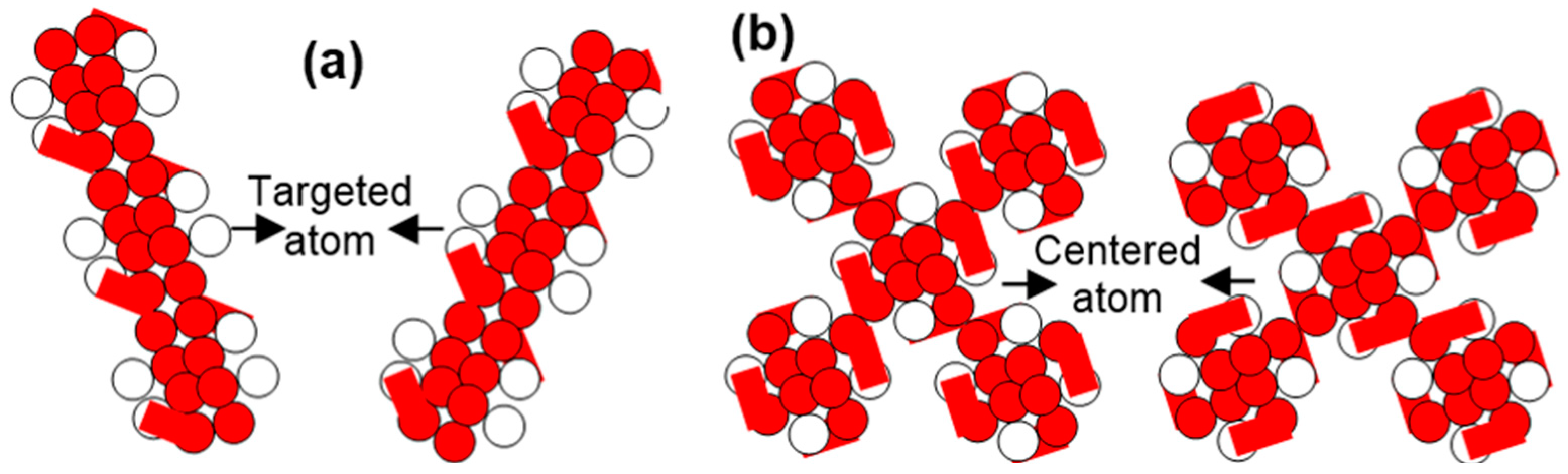
When the gaseous carbon atom converts into the graphite state atom, the engaged forces are mainly related to the surface and space formats. Here, a one-bit energy shape like a dash involves along the left side, and a one-bit energy shape is like a dash along the right side.
The transferring electrons of the graphite atom convert it into the lonsdaleite atom. A bit of dash-shaped energy along the west to south involved, whereas a bit of dash-shaped energy along the east to the south involved. The exerted forces on the transferring electrons remain partially conserved. The exerted forces are related to the surface and grounded formats.
Two electrons are transferred to the nearby positioned unfilled states to convert the lonsdaleite state atom into the diamond atom. As a result, the ground point in the diamond atom goes further below the ground surface than the ground point in the lonsdaleite atom. A carbon atom fully expands under its diamond state.
In the conversion of a carbon atom from one state to any other state, two dash-shaped energy bits involve one along the left side and one along the right side.
Figure 1 (i) shows an electron of a filled state transferring to the nearby unfilled state. Dash-shaped energy is like a pipe through which force can pass. Dash-shaped energy from one end connects to the tip of transferring electron and from the other end connects to the nearby unfilled state.
Figure 1 (i) shows the downward arrow increasing the potential of the electron and the upward arrow decreasing the potential of the electron. In this way, transferring electrons only engage the partially-conserved forces. Forces only exert on the two poles of an electron as its two sides remain hidden during the transfer. An energy bit covers the transferring electron from both sides. Energy bit does not permit force to exert on an electron from those sides.
Therefore, during the transfer, only the forces of two poles exert on the left-sided electron or right-sided electron. The expansion and contraction of a carbon atom under different states depend on the electrons’ potential energy and orientation.
5.0. Estimated hardness of carbon-based materials at Mohs scale
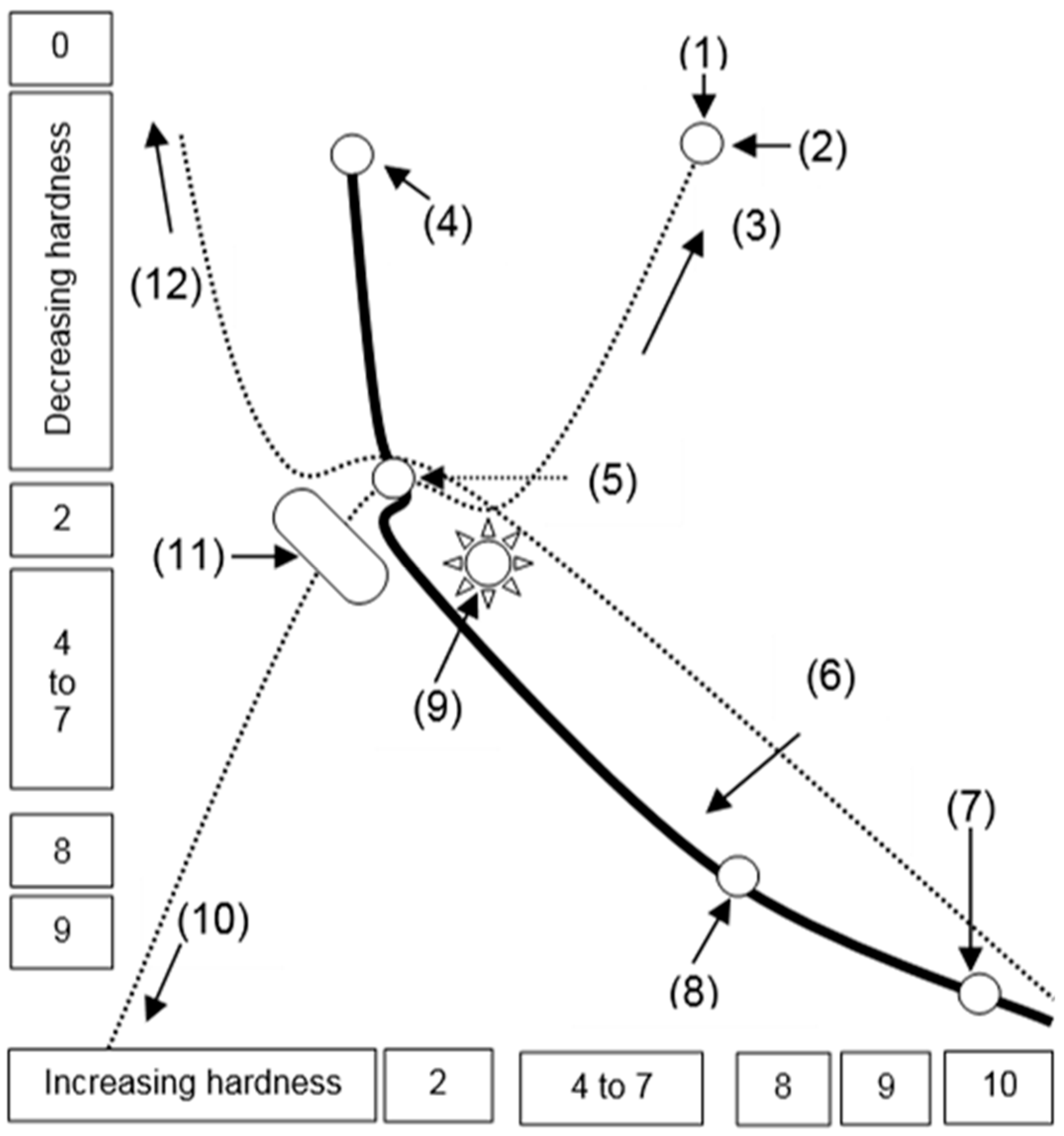
In
Figure 6, the Mohs hardness of different nanostructured and microstructured carbon materials is an estimation. Gaseous carbon atoms keep the hardness zero at the Mohs scale. Structures of graphite, nanotube and fullerene atoms involve partially conserved energy and engage partially conserved force. So, they only measure the average hardness at the Mohs scale. Structures of lonsdaleite, graphene, and glassy carbon involve non-conserved energy and engage non-conserved forces to measure the hardness at a large scale.
The high hardness of lonsdaleite, graphene, and diamond structures is due to the involvement of golf-stick-shaped energy bits. Orientated outer ring electrons undertaking double clamping of positioned energy knots engage a non-conservative force. The same is the case in the structural formation of glassy carbon.
Figure 6 extracts the drawn hardness at the Mohs scale from the published information. However, the published data on the hardness does not consider the energy force detail discussed here. It is an actual need to re-investigate the hardness of different carbon-based materials. A recent study evaluates the hardness of diamond films by nanoindentation [
54]. However, the hardness of carbon films relies on the nature of force and the kind of energy. Pieces of evidence from the published literature and data support the presented models and details here.
Figure 6.
Mohs hardness vs. different carbon allotropes; (1) levitational force at the electron level, (2) graphene, (3) increasing levitational force, (4) gaseous carbon, (5) graphite, (6) increasing gravitational force, (7) diamond, (8) lonsdaleite, (9) fullerene, (10) maximum gravitational force, (11) nanotube, (12) maximum levitational force.
Figure 6.
Mohs hardness vs. different carbon allotropes; (1) levitational force at the electron level, (2) graphene, (3) increasing levitational force, (4) gaseous carbon, (5) graphite, (6) increasing gravitational force, (7) diamond, (8) lonsdaleite, (9) fullerene, (10) maximum gravitational force, (11) nanotube, (12) maximum levitational force.
Undoubtedly, the presented models and hypotheses develop a sense of understanding and new insights into all sorts of carbon materials. It is not possible to cite every study though there are many studies. However, it is an acute need to study carbon films along with different carbon materials not only in structural behavior but also in force energy behavior. The mechanisms of converting one state carbon atom into another state and binding carbon atoms for their structures elucidate new ideas. Thus, all sorts of depositions and syntheses need to optimize.
6.0. Conclusions
None of the carbon states refer to neutral force behavior at the electron level. In the conversion mechanism of a carbon atom from one state to another, two dash-shaped energy bits involve, which transfer electrons to nearby unfilled states. The carbon atom keeps an equilibrium state during the electron transfer mechanism.
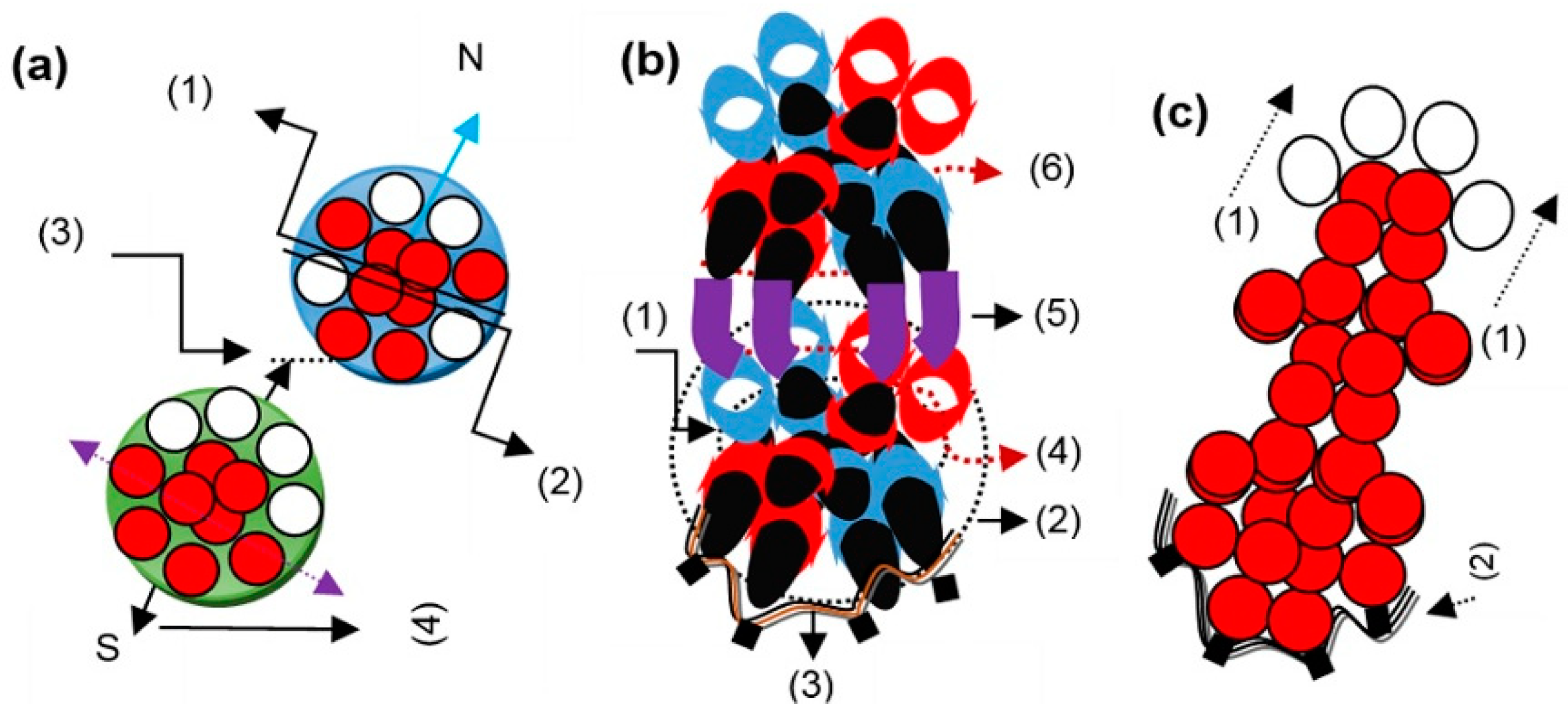
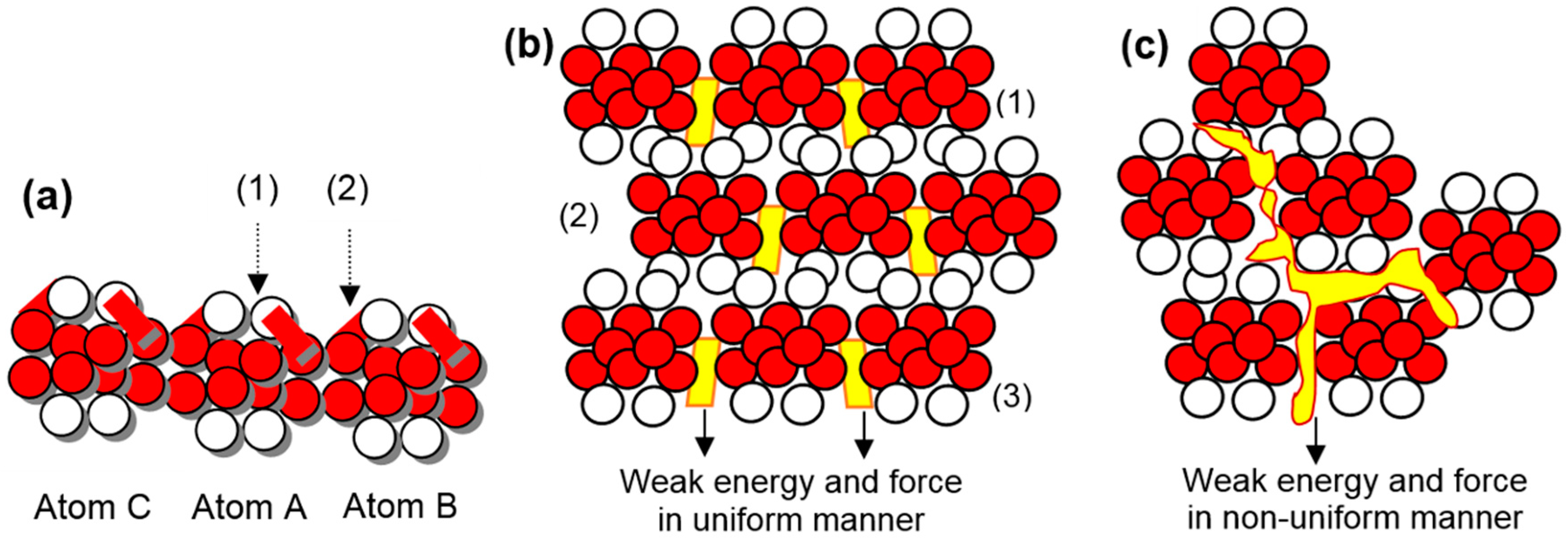
The structural formation is one-dimensional when graphite atoms execute electron dynamics. A structure is two-dimensional when graphite atoms bind by uniformly attained dynamics. Energy force at the atomic level contributes uniformly.
Energy and force, weak in nature, together contribute to binding the graphite state atoms in two dimensions. In the formation or development of an amorphous graphite structure, the energy force at the atomic level contributes non-uniformly.
The structural formation is two-dimensional when nanotube atoms bind by dynamics of suitable outer ring electrons. In a nanotube, electrons of opposite quadrants perform dynamics. In fullerene atoms, the structure is four-dimensional.
In the fullerene structural formation, all four outer ring electrons of the atoms execute dynamics. In the structural formation of graphite in one dimension, nanotube, and fullerene, bits of partially conserved dash-shaped energy involve at the electron level by engaging the partially conserved force, too. Each outer ring electron of the depositing diamond atom undertakes one additional clamping of the outer ring energy knot of the deposited diamond atom. Here, a bit of energy shaped like a golf stick involves transferring the electron up to half-length to another energy knot. The half-length electron above the clamped energy knot remains under the exertion of non-conservative forces.
The binding in diamond state atoms remains from the east-west to the south, so the growth behavior remains from the south pole to the east-west poles. It is a tetra-electron topological structure. The binding in lonsdaleite state atoms remains from the east-west poles to a bit south pole, so the growth behavior remains from a bit south pole to the east-west poles. It is a bi-electron topological structure.
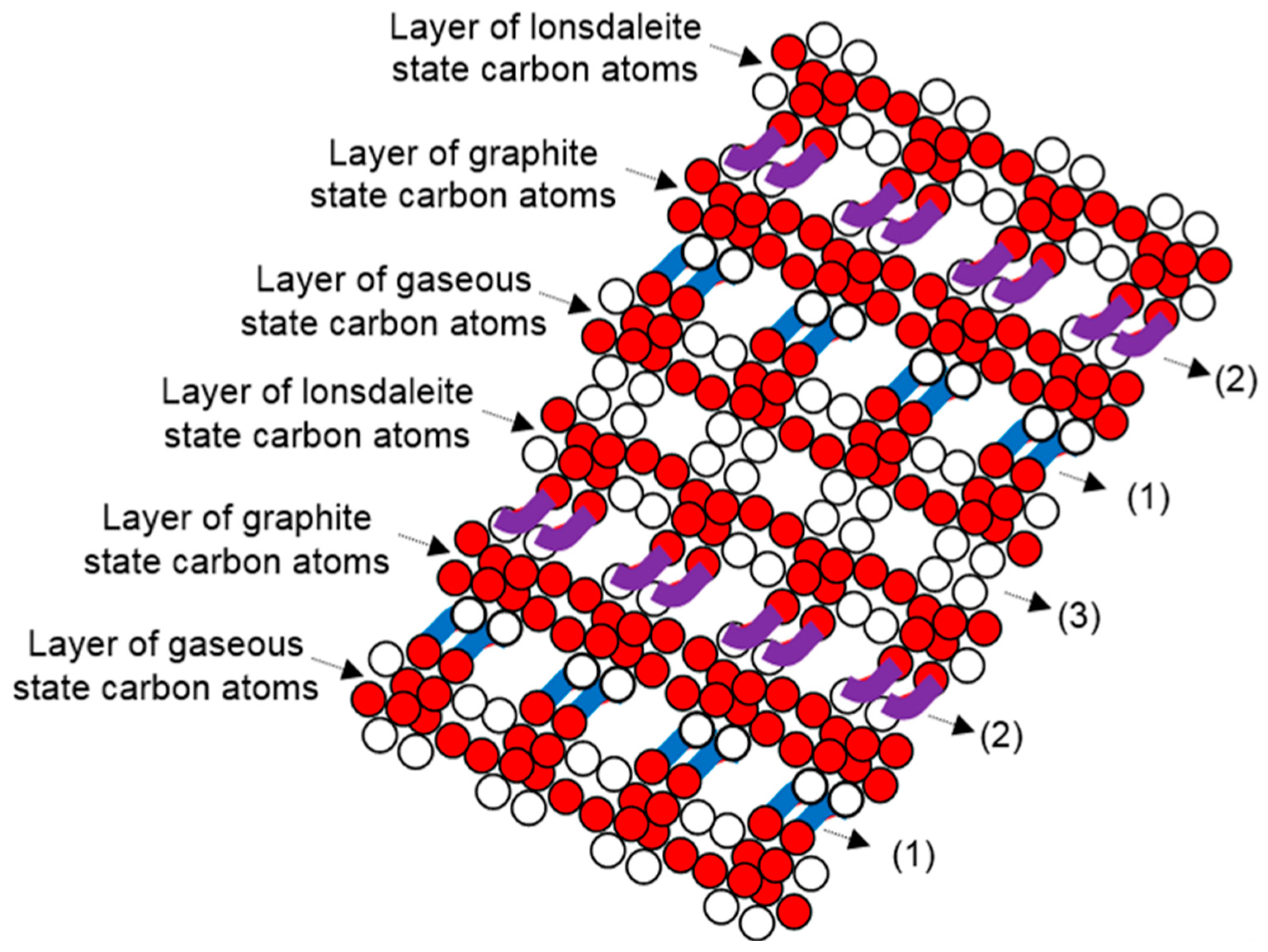
Layers of gaseous, graphite, and lonsdaleite atoms repeat under the same order, and a glassy carbon structure grows. Binding atoms in glassy carbon, the orientated outer ring electrons of the gaseous and lonsdaleite atoms undertake additional clamping of the positioned energy knots of the outer rings of the graphite atoms. In diamond, lonsdaleite, graphene, and glassy carbon structures, bits of non-conserved energy involve at the electron level by engaging the non-conserved force, too.
The hardness of carbon materials relates to the energy force detail at the electronic level. The study of carbon atoms and their structures from the perspective presented here opens new areas of investigation.
Data Availability Statement
This work is based on the fundamental nature of science.
Acknowledgment
The author acknowledges the financial support of all the offices and countries. He is also thankful to whomever he learned from at any stage of his life.
Conflicts of interest
The author declares no conflicts of interest.
References
- M. Ali, I-N. Lin. Forces driving amalgamation of nanoparticles and particles in solution. Forces in Mech., 7 (2022) 100076. [CrossRef]
- M. Ali, Heat and Photon Energy Phenomena: Dealing with Matter at Atomic and Electronic Level. (2022), https://www.preprints.org/manuscript/201701.0028/v14.
- M. Ali, Atoms in Gaseous and Solid States and their Energy and Force Relationships under Transitional Behaviors. (2023). [CrossRef]
- M. Ali, Structure Evolutions in Atoms of the Elements Executing Confined Interstate Electron Dynamics. (2023), http://arxiv.org/abs/1611.01255v31.
- M. Ali, Atoms of None of the Elements Ionize While Atoms of Inert Behavior Split by Photonic Current. (2022), http://arxiv.org/abs/1611.05392v31.
- M. Ali, I-N. Lin, Phase transitions and critical phenomena of tiny grains carbon films synthesized in microwave-based vapor deposition system. Surf. Interface Anal. 51 (2019) 389-399. [CrossRef]
- M. Ali, M. Ürgen, Switching dynamics of morphology-structure in chemically deposited carbon films –A new insight, Carbon 122 (2017) 653-663. [CrossRef]
- M. Ali, Etching of Photon Energy into Binding Energy in Depositing Carbon Films at Different Chamber Pressures. (2022), https://arxiv.org/abs/1802.00730v24. [CrossRef]
- M. Ali, M. Ürgen, Simultaneous growth of diamond and nanostructured graphite thin films by hot filament chemical vapor deposition, Solid State Sci. 14 (2012) 150-154. [CrossRef]
- M. Ali, I-N. Lin, Gold Nanostructures and Microstructures with Tunable Aspect Ratios for High-Speed Uni- and Multidirectional Photonic Applications. ACS Appl. Nano Mater. 3 (9) (2020) 9410-9424. [CrossRef]
- Y. Gao, et al., Ultrahard carbon film from epitaxial two-layer graphene, Nature Nanotechnol. 13 (2018) 133-138. [CrossRef]
- J.-B. Wu, M. L. Lin, X. Cong, H. N. Liu, P. H. Tan, Raman spectroscopy of graphene-based materials and its applications in related devices, Chem. Soc. Rev. 47 (2018) 1822-1873. [CrossRef]
- R. Shoukat, M. I. Khan, Synthesis of vertically aligned carbon nanofibers using inductively coupled plasma-enhanced chemical vapor deposition, Electr. Eng., 100 (2018) 997-1002. [CrossRef]
- M. S. Cao, et al., Graphene nanohybrids: excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves, J. Mater. Chem. C, 6 (2018) 4586-4602. [CrossRef]
- L. Fu, et al., A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol, Microchimica Acta 185 (2018) 87. [CrossRef]
- D. Y. Hu, J. X. Hu, H. L. Jiang, J. Xu, A highly effective energy mitigation system combining carbon nanotube and buckyballs, Eur. Phys. J.-Spec. Top. 127 (2018) 155-166. [CrossRef]
- C. P. Hsu, et al., Buckball-, carbon nanotube-, graphite-, and graphene-enhanced dehydrogenation of lithium, Chem. Commun. 49 (2013) 8845-8847. [CrossRef]
- C. Y. Chen, et al., Laser writing of coherent colour centres in diamond, Nature Photonics 11 (2017) 77-80.
- Z. Liu, et al., Graphite-graphene architecture stabilizing ultrafine Co3O4nanoparticles for superior oxygen evolution, Carbon 140 (2018) 17-23. [CrossRef]
- Taylor, et al., Precursor gas composition optimisation for large area boron doped nano-crystalline diamond growth by MW-LA-PECVD, Carbon 128 (2018) 164-171. [CrossRef]
- X. Cheng, W. J. Zong, Anisotropic evolution of damaged carbons of a mechanically polished diamond surface in low-temperature annealing, Diam. Relat. Mater. 90 (2018) 7-17. [CrossRef]
- M. Maruyama, S. Okada, Geometric and electronic structure of a two-dimensional covalent network of sp2 and sp3 carbon atoms, Diam. Relat. Mater. 81 (2018) 103-107. [CrossRef]
- Narjabadifam, F. Vakili-Tahami, M. Zehsaz, Elastic and failure properties of carbon nanocones using molecular dynamics simulation, Fuller. Nanotub. Carbon Nanostruct. 26 (2018) 777-789. [CrossRef]
- T. M. A. Rahman, et al., Burning and graphitization of optically levitated nanodiamonds in vacuum, Sci. Rep. 6 (2016) 21633. [CrossRef]
- C. Frangeskou, et al., Pure nanodiamonds for levitated optomechanics in vacuum, New J. Phys. 20 (2018) 043016. [CrossRef]
- L. Yang, et al., Effect of heat treatment on mechanical property of amorphous carbon films by magnetron sputtering, Diam. Relat. Mater. 129 (2022) 109328. [CrossRef]
- J. -Q. Huang, et al., A freestanding hydroxylated carbon nanotube film boosting the stability of Zn metal anodes, Mater. Today Commun. 32 (2022) 103939.
- J. Shi, R. Zhao, S. Ke, W. Wang, C. Wang, Probing the ultra-low friction mechanism of hydrogenated carbon films with controllable fullerene-like nano-structures grown with different bias voltage, Tribol. Int., 175 (2022) 107796. [CrossRef]
- T. Ishii, D. Okuhara, R. Kobayashi, J. -ichi Ozaki, Modulation of the electronic state of carbon thin films by inorganic substrates, Carbon, 196 (2022) 313-319.
- Shirani, et al., Mechanochemically driven formation of protective carbon films from ethanol environment, Mater. Today Chem., 26 (2022) 101112. [CrossRef]
- H. Bae, K. Sasai, H. Suzuki, H. Toyoda, High-speed deposition of graphite-like carbon film by Ar/C6H6 surface-wave plasma with high-voltage pulse biasing, Vacuum, 192 (2021) 110429. [CrossRef]
- Thakur, et al., Investigating the effect of thickness on the structural and magnetic properties of carbon thin film, Carbon, 191 (2022) 205-214. [CrossRef]
- J. Jia, et al., Structure and Electrochemical Properties of Carbon Films Prepared by a Electron Cyclotron Resonance Sputtering Method, Anal. Chem. 79 (2007) 98-105. [CrossRef]
- J. Wang, et al., Alternative Friction Mechanism for Amorphous Carbon Films Sliding against Alumina, Ind. Eng. Chem. Res. 58 (2019) 4810-4817. [CrossRef]
- J. H. Kim, et al., Tailored Hydrogen-Free Carbon Films by Tuning the sp2/sp3 Configuration, ACS Appl. Electron. Mater. 3 (2021) 1771-1779.
- Y. Kato, et al., Indentation behavior of suspended single-walled carbon nanotube films, Carbon Trends, 5 (2021) 100112. [CrossRef]
- Y. Liao, et al., Single-Walled Carbon Nanotube Thin Film with High Semiconducting Purity by Aerosol Etching toward Thin-Film Transistors, ACS Appl. Nano Mater., 4 (2021) 9673-9679. [CrossRef]
- H. Zhan, et al., Highly aligned and densified carbon nanotube films with superior thermal conductivity and mechanical strength, Carbon, 186 (2022) 205-214. [CrossRef]
- W. Larson, et al., Arresting Photodegradation in Semiconducting Single-Walled Carbon Nanotube Thin Films, ACS Appl. Nano Mater., 5 (2022) 3502-3511. [CrossRef]
- M. Zhang, T. Xie, X. Qian, Y. Zhu, X. Liu, Mechanical Properties and Biocompatibility of Ti-doped Diamond-like Carbon Films, ACS Omega, 5 (2020) 22772-22777. [CrossRef]
- Z. Zhai, H. Shen, J. Chen, X. Li, Y. Jiang, Evolution of Structural and Electrical Properties of Carbon Films from Amorphous Carbon to Nanocrystalline Graphene on Quartz Glass by HFCVD, ACS Appl. Mater. Interfaces, 10 (2018) 17427-17436. [CrossRef]
- J. Li, et al., In Situ Fabrication of Magnetic and Hierarchically Porous Carbon Films for Efficient Electromagnetic Wave Shielding and Absorption, ACS Appl. Mater. Interfaces, 14 (2022) 33675-33685. [CrossRef]
- R. Wang, et al., Precisely Controlled Vertical Alignment in Mesostructured Carbon Thin Films for Efficient Electrochemical Sensing, ACS Nano, 15 (2021) 7713-7721. [CrossRef]
- S. Wang, A. Roy, K. Komvopoulos, Thermal stability and diffusion characteristics of ultrathin amorphous carbon films grown on crystalline and nitrogenated silicon substrates by filtered cathodic vacuum arc deposition, Sci. Rep., 11 (2021) 13106. [CrossRef]
- M. Ali, Sleeping under Trees at Night is not Healthful; Sleeping during the Day is Healthful – Forming CO (CO2) and their Dissociation. (2022). [CrossRef]
- W. H. Zachariasen, The atomic arrangement in glass, J. Am. Chem. Soc., 54 (1932) 3841-3851. [CrossRef]
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 354 (1991) 56-58. [CrossRef]
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, R. E. Smalley, C60: Buckminsterfullerene, Nature, 318 (1985) 162-163. [CrossRef]
- Q. Chen, Investigation of Diamond Nucleation under Very Low Pressure in Chemical Vapor Deposition. (1997), arXiv:cond-mat/9708146.
- Cammarata, M. Kaintz, T. Polcar, Engineering width and directness of the band gap in diamond-based materials: An ab initio investigation towards electron-structure features control, Diam. Relat. Mater., 128 (2022) 109237. [CrossRef]
- P. Németh, et al., Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material, Nat. Commun., 5 (2014) 5447. [CrossRef]
- K. Geim, Graphene prehistory, Phys. Scr., 2012 (2012) 014003.
- V. Uskoković, A historical review of glassy carbon: Synthesis, structure, properties and applications, Carbon Trends, 5 (2021) 100116. [CrossRef]
- J. Xiong, et al., Mechanical properties evaluation of diamond films via nanoindentation, Diam. Relat. Mater., 130 (2022) 109403. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Author’s biography:
 |
Mubarak Ali graduated from the “University of the Punjab” with BSc (Phys & Math) in 1996 and MSc in Materials Science with distinction from Bahauddin Zakariya University, Multan, Pakistan (1998); his thesis work was completed at Quaid-i-Azam University Islamabad. He gained a Ph.D. in Mechanical Engineering from the Universiti Teknologi Malaysia under the award of the Malaysian Technical Cooperation Programme (MTCP;2004-07) and a postdoc in advanced surface technologies at Istanbul Technical University under the foreign fellowship of The Scientific and Technological Research Council of Turkey (TÜBİTAK, 2010). Dr. Mubarak completed another postdoc in nanotechnology at the Tamkang University Taipei, 2013-2014, sponsored by National Science Council, now M/o Science and Technology, Taiwan (R.O.C.). Presently, he is working as an Assistant Professor on the tenure track at COMSATS University Islamabad (previously known as COMSATS Institute of Information Technology), Islamabad, Pakistan (since May 2008, and the position renewal is in the process) and before that, worked as assistant director/deputy director at M/o Science & Technology (Pakistan Council of Renewable Energy Technologies, Islamabad, 2000-2008). The Institute for Materials Research, Tohoku University, Japan, invited him to deliver a scientific talk. He gave several scientific talks in various countries. His core area of research includes materials science, physics & nanotechnology. He was also offered a merit scholarship for Ph.D. study by the Higher Education Commission, Government of Pakistan, but he did not avail himself of the opportunity. He earned a diploma (in English) and a certificate (in the Japanese language) in 2000 and 2001, respectively, part-time from the National University of Modern Languages, Islamabad. He is the author of several articles available at the following links; https://www.researchgate.net/profile/Mubarak_Ali5 & https://scholar.google.com.pk/citations?hl=en&user=UYjvhDwAAAAJ
|
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).