Submitted:
09 March 2024
Posted:
13 March 2024
You are already at the latest version
Abstract
Keywords:
1.0. Introduction
2.0. Experimental details
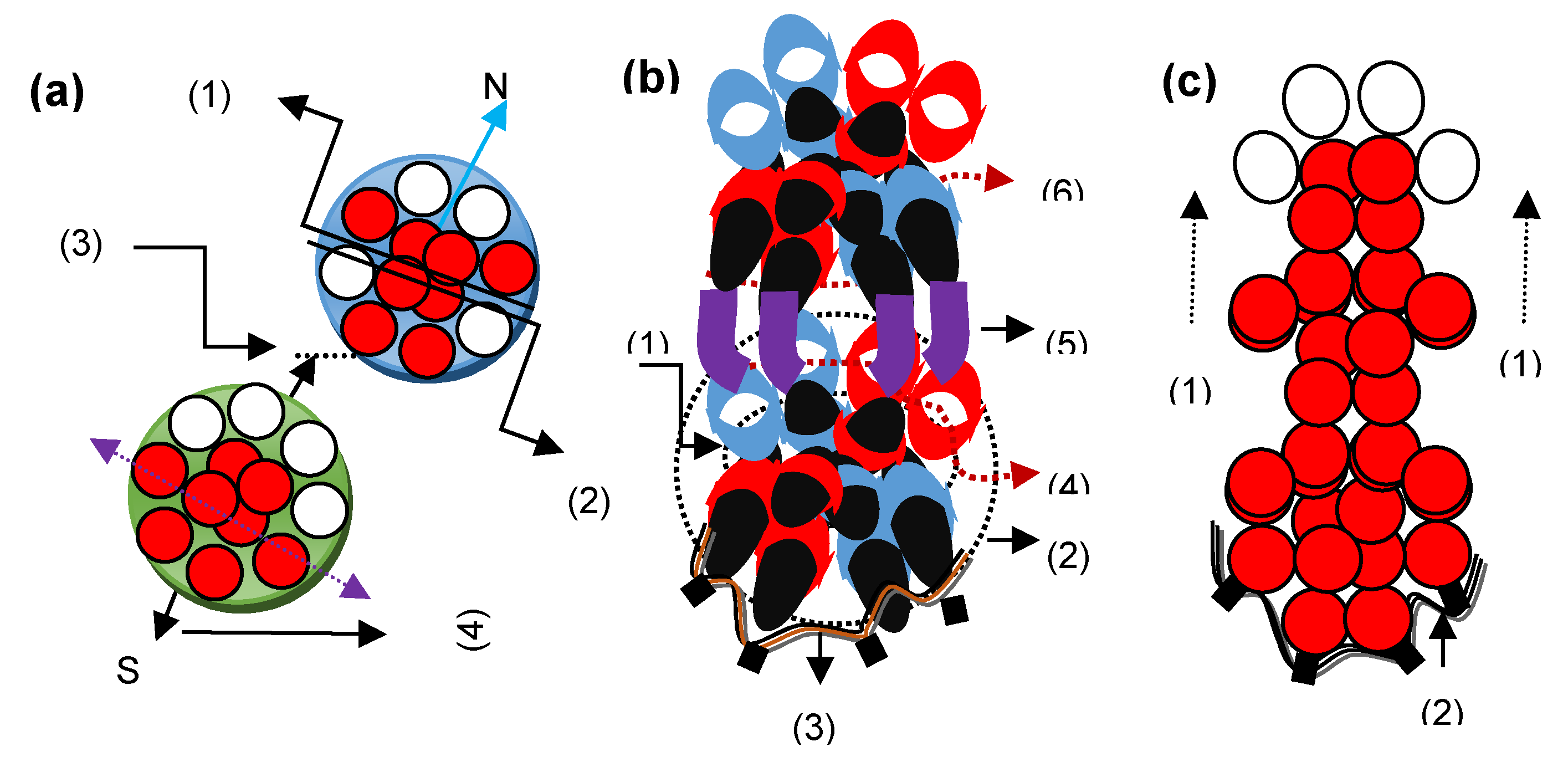
3.0. Atomic structure in the carbon and electron transfer mechanism
3.1. Carbon lattice and structure of carbon allotropes or states
3.2. Electron transfer mechanism in a carbon atom
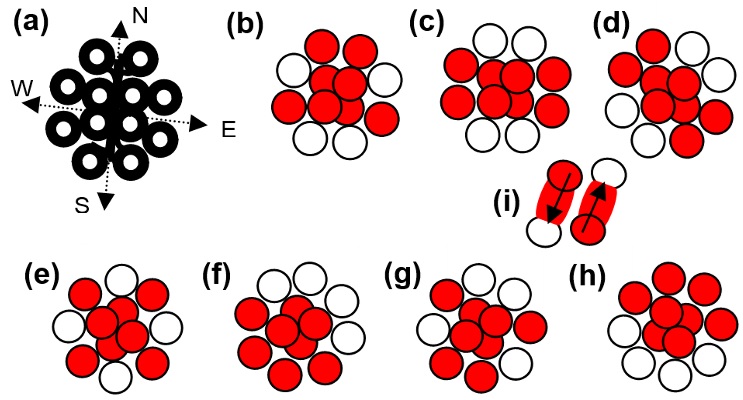
4.0. Structural formations in different state carbon atoms
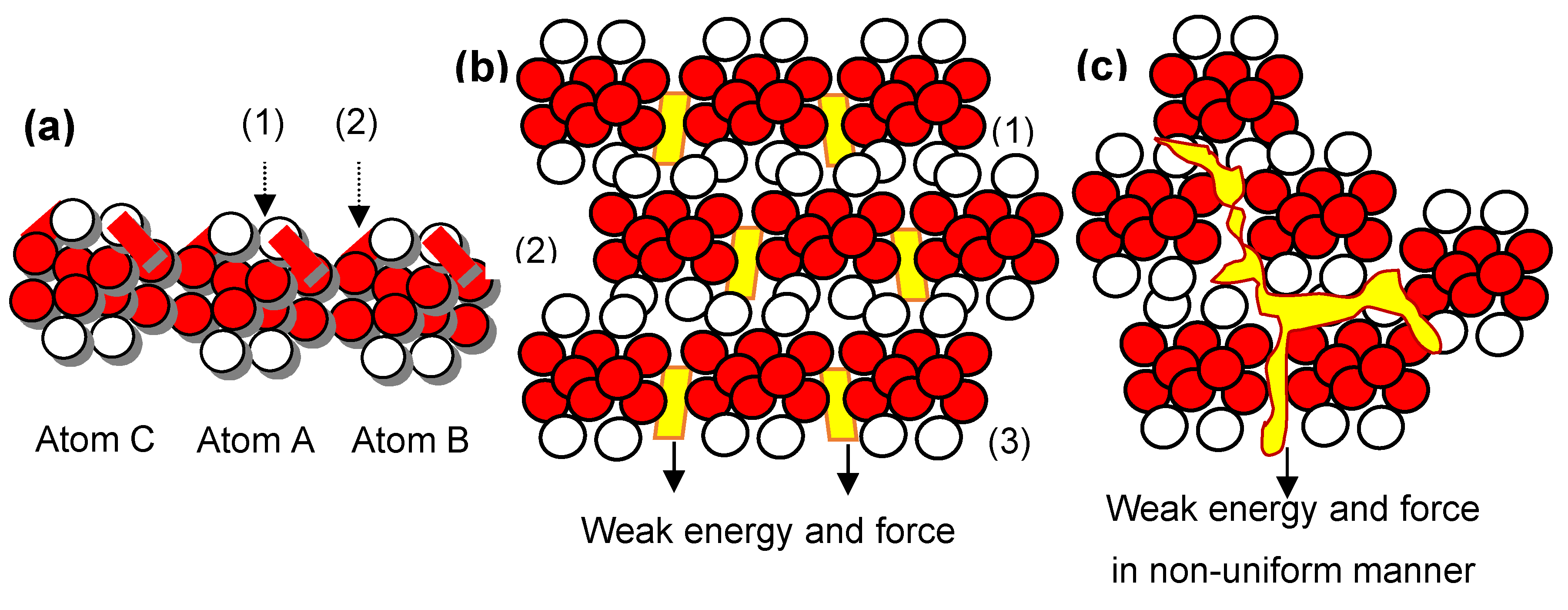
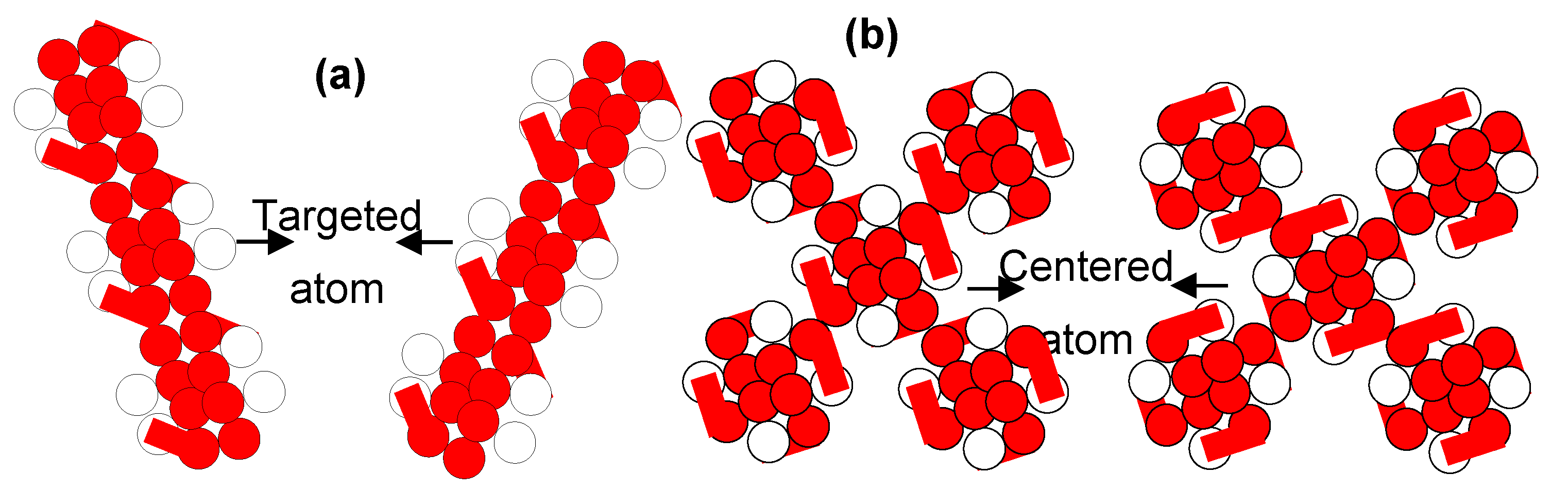
4.1. Structures of graphite atoms
4.1.1. Graphite structure under the electron dynamics of atoms
4.1.2. Graphite structure under the attained dynamics of atoms
4.1.3. Amorphous graphite structure
4.2. Formation of nanotube and fullerene structures
4.3. Formation of a diamond structure
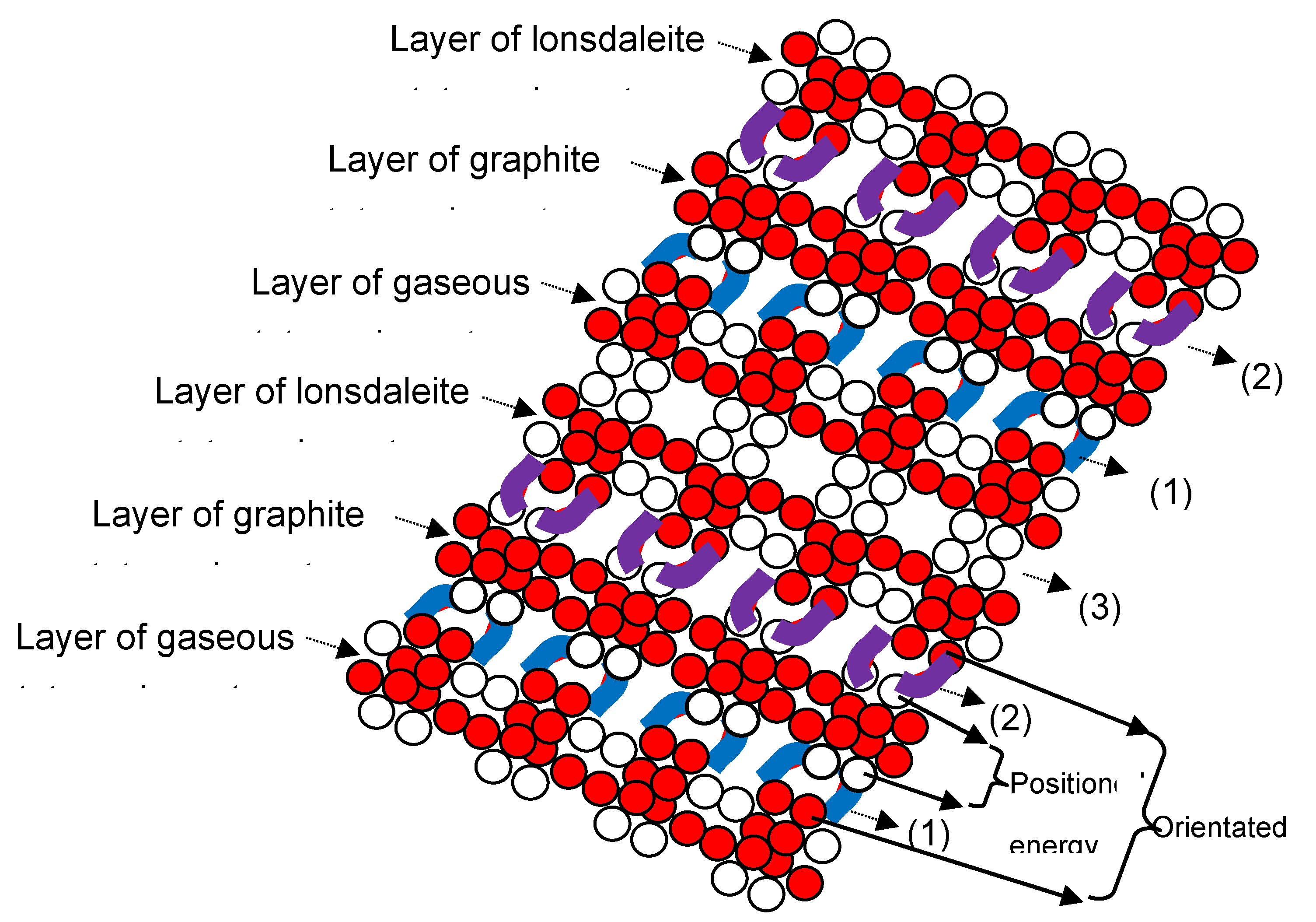
4.4. Lonsdaleite and graphene structures
4.5. Formation of glassy carbon structure
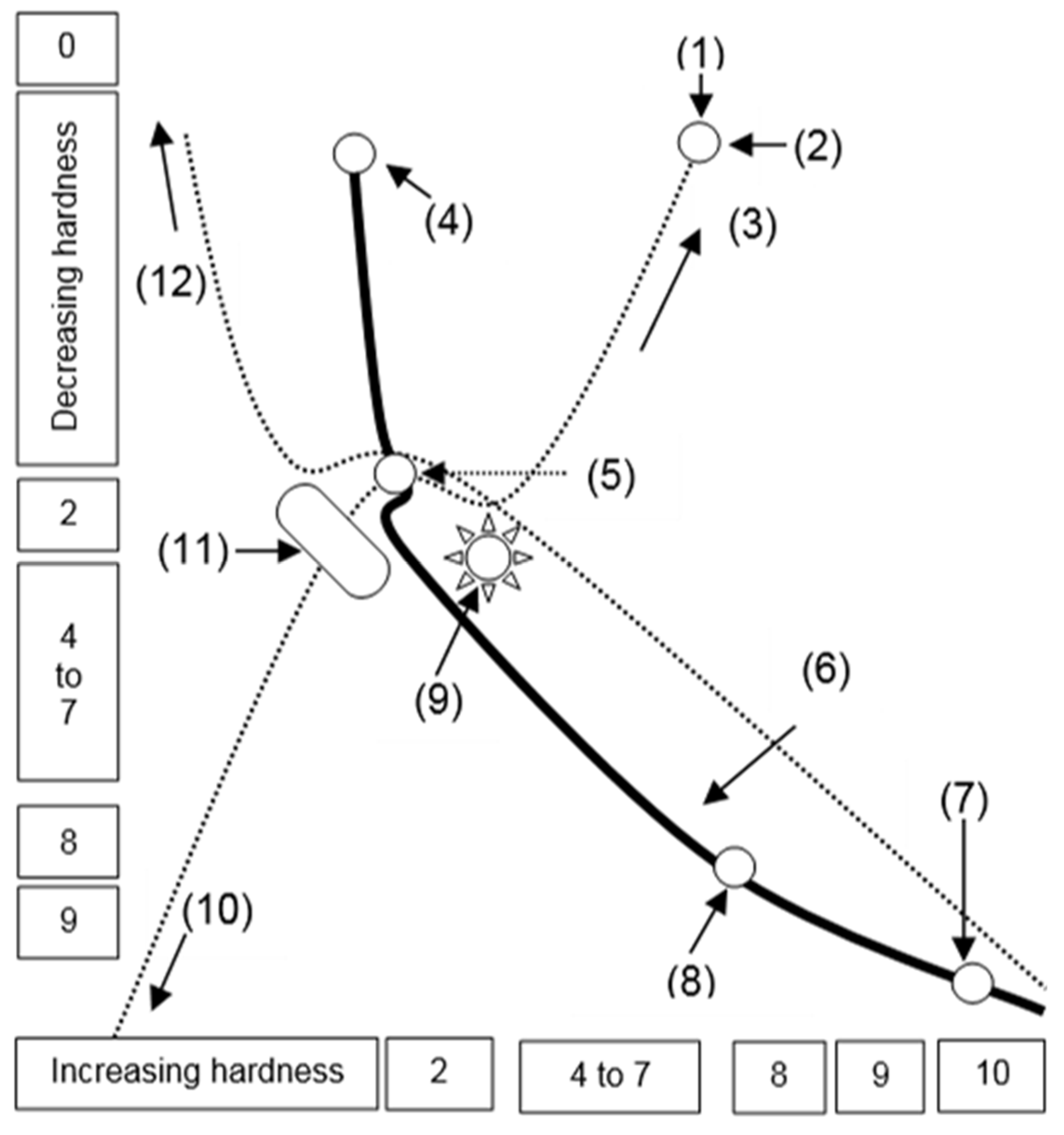
5.0. Estimated hardness of carbon-based materials at the Mohs scale
6.0. Conclusion
Data availability statement
Acknowledgments
Conflicts of interest
References
- Ali, M.; Lin, I.-N. Forces driving amalgamation of nanoparticles and particles in solution. Forces in Mech. 2022, 7, 100076. [Google Scholar] [CrossRef]
- Ali, M. Heat and Photon Energy Phenomena: Dealing with Matter at the Atomic and Electronic Levels. 2024. https://www.preprints.org/manuscript/201701. 0028. [Google Scholar]
- Ali, M. Atoms in Gaseous and Solid States and their Energy and Force Relationships under Transitional Behaviors. 2023. [CrossRef]
- Ali, M. Structure Evolutions in Atoms of the Elements Executing Confined Interstate Electron Dynamics. 2023. http://arxiv.org/abs/1611. 0125. [Google Scholar]
- Ali, M. Atoms of None of the Elements Ionize While Atoms of Inert Behavior Split by Photonic Current. 2024. http://arxiv.org/abs/1611. 0539. [Google Scholar]
- Ali, M.; Lin, I.-N. Phase transitions and critical phenomena of tiny grains carbon films synthesized in microwave-based vapor deposition system. Surf. Interface Anal. 2019, 51, 389–399. [Google Scholar] [CrossRef]
- Ali, M.; Ürgen, M. Switching dynamics of morphology-structure in chemically deposited carbon films –A new insight. Carbon 2017, 122, 653–663. [Google Scholar] [CrossRef]
- Ali, M. Etching of Photon Energy into Binding Energy in Depositing Carbon Films at Different Chamber Pressures. J. Mater. Sci. Mater. Electron. 2023, 34, 1209. [Google Scholar] [CrossRef]
- Ali, M.; Ürgen, M. Simultaneous growth of diamond and nanostructured graphite thin films by hot filament chemical vapor deposition. Solid State Sci. 2012, 14, 150–154. [Google Scholar] [CrossRef]
- Ali, M.; Lin, I.-N. Gold Nanostructures and Microstructures with Tunable Aspect Ratios for High-Speed Uni- and Multidirectional Photonic Applications. ACS Appl. Nano Mater. 2020, 3, 9410–9424. [Google Scholar]
- Gao, Y.; et al. Ultrahard carbon film from epitaxial two-layer graphene. Nature Nanotechnol. 2018, 13, 133–138. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Synthesis of vertically aligned carbon nanofibers using inductively coupled plasma-enhanced chemical vapor deposition. Electr. Eng. 2018, 100, 997–1002. [Google Scholar] [CrossRef]
- Cao, M.S.; et al. Graphene nanohybrids: excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves. J. Mater. Chem. C 2018, 6, 4586–4602. [Google Scholar] [CrossRef]
- Fu, L.; et al. A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchimica Acta 2018, 185, 87. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.Y.; Hu, J.X.; Jiang, H.L.; Xu, J. A highly effective energy mitigation system combining carbon nanotube and buckyballs. Eur. Phys. J.-Spec. Top. 2018, 127, 155–166. [Google Scholar] [CrossRef]
- Hsu, C.P.; et al. Buckball-, carbon nanotube-, graphite-, and graphene-enhanced dehydrogenation of lithium. Chem. Commun. 2013, 49, 8845–8847. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; et al. Laser writing of coherent colour centres in diamond. Nature Photonics 2017, 11, 77–80. [Google Scholar] [CrossRef]
- Z. Liu, et al. Graphite-graphene architecture stabilizing ultrafine Co3O4 nanoparticles for superior oxygen evolution. Carbon 2018, 140, 17–23. [Google Scholar] [CrossRef]
- Taylor, A.; et al. Precursor gas composition optimisation for large area boron doped nano-crystalline diamond growth by MW-LA-PECVD. Carbon 2018, 128, 164–171. [Google Scholar] [CrossRef]
- Cheng, X.; Zong, W.J. Anisotropic evolution of damaged carbons of a mechanically polished diamond surface in low-temperature annealing. Diam. Relat. Mater. 2018, 90, 7–17. [Google Scholar] [CrossRef]
- Maruyama, M.; Okada, S. Geometric and electronic structure of a two-dimensional covalent network of sp2 and sp3 carbon atoms. Diam. Relat. Mater. 2018, 81, 103–107. [Google Scholar] [CrossRef]
- Narjabadifam, F. Vakili-Tahami, M. Zehsaz, Elastic and failure properties of carbon nanocones using molecular dynamics simulation. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 777–789. [Google Scholar] [CrossRef]
- Rahman, A.T.M.A.; et al. Burning and graphitization of optically levitated nanodiamonds in vacuum. Sci. Rep. 2016, 6, 21633. [Google Scholar] [CrossRef]
- Frangeskou, A.C.; et al. Pure nanodiamonds for levitated optomechanics in vacuum. New, J. Phys. 2018, 20, 043016. [Google Scholar] [CrossRef]
- Yang, L.; et al. Effect of heat treatment on mechanical property of amorphous carbon films by magnetron sputtering. Diam. Relat. Mater. 2022, 129, 109328. [Google Scholar] [CrossRef]
- Huang, J.-Q.; et al. A freestanding hydroxylated carbon nanotube film boosting the stability of Zn metal anodes. Mater. Today Commun. 2022, 32, 103939. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, R.; Ke, S.; Wang, W.; Wang, C. Probing the ultra-low friction mechanism of hydrogenated carbon films with controllable fullerene-like nanostructures grown with different bias voltage. Tribol. Int. 2022, 175, 107796. [Google Scholar] [CrossRef]
- Ishii, T.; Okuhara, D.; Kobayashi, R.; Ozaki, J.-I. Modulation of the electronic state of carbon thin films by inorganic substrates. Carbon 2022, 196, 313–319. [Google Scholar] [CrossRef]
- Shirani, A.; et al. Mechanochemically driven formation of protective carbon films from ethanol environment. Mater. Today Chem. 2022, 26, 101112. [Google Scholar] [CrossRef]
- Bae, H.; Sasai, K.; Suzuki, H.; Toyoda, H. High-speed deposition of graphite-like carbon film by Ar/C6H6 surface-wave plasma with high-voltage pulse biasing. Vacuum 2021, 192, 110429. [Google Scholar] [CrossRef]
- Thakur, B.; et al. Investigating the effect of thickness on the structural and magnetic properties of carbon thin film. Carbon 2022, 191, 205–214. [Google Scholar] [CrossRef]
- Jia, J.; et al. Structure and Electrochemical Properties of Carbon Films Prepared by an Electron Cyclotron Resonance Sputtering Method. Anal. Chem. 2007, 79, 98–105. [Google Scholar] [CrossRef]
- Wang, J.; et al. Alternative Friction Mechanism for Amorphous Carbon Films Sliding against Alumina. Ind. Eng. Chem. Res. 2019, 58, 4810–4817. [Google Scholar] [CrossRef]
- Kim, J.H.; et al. Tailored Hydrogen-Free Carbon Films by Tuning the sp2/sp3 Configuration. ACS Appl. Electron. Mater. 2021, 3, 1771–1779. [Google Scholar]
- Kato, Y.; et al. Indentation behavior of suspended single-walled carbon nanotube films. Carbon Trends 2021, 5, 100112. [Google Scholar] [CrossRef]
- Liao, Y.; et al. Single-Walled Carbon Nanotube Thin Film with High Semiconducting Purity by Aerosol Etching toward Thin-Film Transistors. ACS Appl. Nano Mater. 2021, 4, 9673–9679. [Google Scholar]
- Zhan, H.; et al. Highly aligned and densified carbon nanotube films with superior thermal conductivity and mechanical strength. Carbon 2022, 186, 205–214. [Google Scholar] [CrossRef]
- Larson, B.W.; et al. Arresting Photodegradation in Semiconducting Single-Walled Carbon Nanotube Thin Films. ACS Appl. Nano Mater. 2022, 5, 3502–3511. [Google Scholar]
- Zhang, M.; Xie, T.; Qian, X.; Zhu, Y.; Liu, X. Mechanical Properties and Biocompatibility of Ti-doped Diamond-like Carbon Films. ACS Omega 2020, 5, 22772–22777. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J.; Li, X.; Jiang, Y. Evolution of Structural and Electrical Properties of Carbon Films from Amorphous Carbon to Nanocrystalline Graphene on Quartz Glass by HFCVD. ACS Appl. Mater. Interfaces 2018, 10, 17427–17436. [Google Scholar] [CrossRef]
- Li, J.; et al. In Situ Fabrication of Magnetic and Hierarchically Porous Carbon Films for Efficient Electromagnetic Wave Shielding and Absorption. ACS Appl. Mater. Interfaces 2022, 14, 33675–33685. [Google Scholar] [CrossRef]
- Wang, R.; et al. Precisely Controlled Vertical Alignment in Mesostructured Carbon Thin Films for Efficient Electrochemical Sensing. ACS Nano 2021, 15, 7713–7721. [Google Scholar] [CrossRef]
- Wang, S.; Roy, A.; Komvopoulos, K. Thermal stability and diffusion characteristics of ultrathin amorphous carbon films grown on crystalline and nitrogenated silicon substrates by filtered cathodic vacuum arc deposition. Sci. Rep. 2021, 11, 13106. [Google Scholar] [CrossRef]
- Ali, M. Formation of CO and CO2 molecules under the tree roof and their dissociation. 2023. [CrossRef]
- Li, F.N.; Bao, H.W.; Li, Y.; Ma, F.; Wang, H.X. Laser-induced diamond/graphite structure for all-carbon deep-ultraviolet photodetector. Appl. Surf. Sci. 2023, 636, 157818. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Al Tahhan, A.B.; Alkhedher, M.; Mourad, A.-H.I.; Ramadan, M.; Nawash, J.M. Effect of induced vacancy defects on the mechanical behavior of wavy single-walled carbon nanotubes. Nano Trends 2023, 3, 100016. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Ali, M. Qualitative analyses of thin film-based materials validating new structures of atoms. Mater. Today Commun. 2023, 36, 106552. [Google Scholar] [CrossRef]
- Chen, Q. Investigation of Diamond Nucleation under Very Low Pressure in Chemical Vapor Deposition. 1997. arXiv:cond-mat/9708146.
- Cammarata, A.; Kaintz, M.; Polcar, T. Engineering width and directness of the band gap in diamond-based materials: An ab initio investigation towards electron-structure features control. Diam. Relat. Mater. 2022, 128, 109237. [Google Scholar] [CrossRef]
- Han, Q.; Luo, K.; Gao, Q.; Wu, Y.; He, J. Theoretical study on phase transition of various graphitic structures under high pressure. Diam. Related Mater. 2023, 33, 109725. [Google Scholar] [CrossRef]
- Li, M.; et al. Effect of pressure on large size diamond single crystal synthesized by temperature gradient method under low nitrogen condition. Int. J. Refract. Met. Hard Mater. 2023, 115, 106307. [Google Scholar] [CrossRef]
- Németh, P.; et al. Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material. Nat. Commun. 2014, 5, 5447. [Google Scholar] [CrossRef]
- Geim, K. Graphene prehistory. Phys. Scr. 2012, 2012, 014003. [Google Scholar] [CrossRef]
- Uskoković, V. A historical review of glassy carbon: Synthesis, structure, properties and applications. Carbon Trends 2021, 5, 100116. [Google Scholar] [CrossRef]
- Kim, G.; Noh, S.; Lee, S.; Lee, K.-S.; Kim, K.-J. In situ observation of an atomically modified glassy carbon surface: From synthesis of an Fe-N-C catalyst to intrinsic combined reactions of hydrogen dissociation and the oxygen reduction reaction. Diam. Relat. Mater. 2022, 130, 109464. [Google Scholar] [CrossRef]
- Xiong, J. et al.; et al. Mechanical properties evaluation of diamond films via nanoindentation. Diam. Relat. Mater. 2022, 130, 109403. [Google Scholar] [CrossRef]
- Ali, M. Structural analyses of carbon films deposited at different total mass rates in hot-filament CVD system. Mater. Adv. 2023, 4, 5361–5370. [Google Scholar] [CrossRef]
Bibliographic detail
 |
| In 1996, Mubarak Ali earned a B.Sc. degree in Physics and Mathematics. The University of the Punjab awarded him a degree. His M.Sc. degree in Materials Science in 1998. Bahauddin Zakariya University Multan awarded him a master’s degree with distinction. He completed his thesis at Quaid-i-Azam University Islamabad. He gained a PhD in Mechanical Engineering from the Universiti Teknologi Malaysia under the award of the Malaysian Technical Cooperation Programme (MTCP;2004-07) and a postdoc in advanced surface technologies at Istanbul Technical University under the foreign fellowship of The Scientific and Technological Research Council of Turkey (TÜBİTAK, 2010). Dr Mubarak completed another postdoc in nanotechnology at Tamkang University Taipei, 2013-2014, sponsored by the National Science Council, now the Ministry of Science and Technology, Taiwan. He remained working as an Assistant Professor on the tenure track at COMSATS University Islamabad from May 2008 to June 2018, previously known as the COMSATS Institute of Information Technology. His new position is in process. He also worked as an assistant director and deputy director at M/o Science & Technology, Pakistan Council of Renewable Energy Technologies, Islamabad, from January 2000 to May 2008. The Institute for Materials Research at Tohoku University Japan invited Dr. Mubarak to deliver a scientific talk. His scientific research has been a part of many conferences organized by renowned universities in many countries. His core areas of research include materials science, physics, surface and coating technology, carbon-based materials, materials engineering, materials chemistry, physical chemistry, sustainability, energy science, and nanotechnology. He also won a merit scholarship for PhD studies from the Higher Education Commission, Government of Pakistan. However, he did not obtain this opportunity. He earned a diploma (in English) and a certificate (in the Japanese language) in 2000 and 2001, respectively, part-time from the National University of Modern Languages, Islamabad. He is the author of several articles. Please refer to the link https://www.researchgate.net/profile/Mubarak_Ali5 and the link https://scholar.google.com.pk/citations?hl=en&user=UYjvhDwAAAAJ |
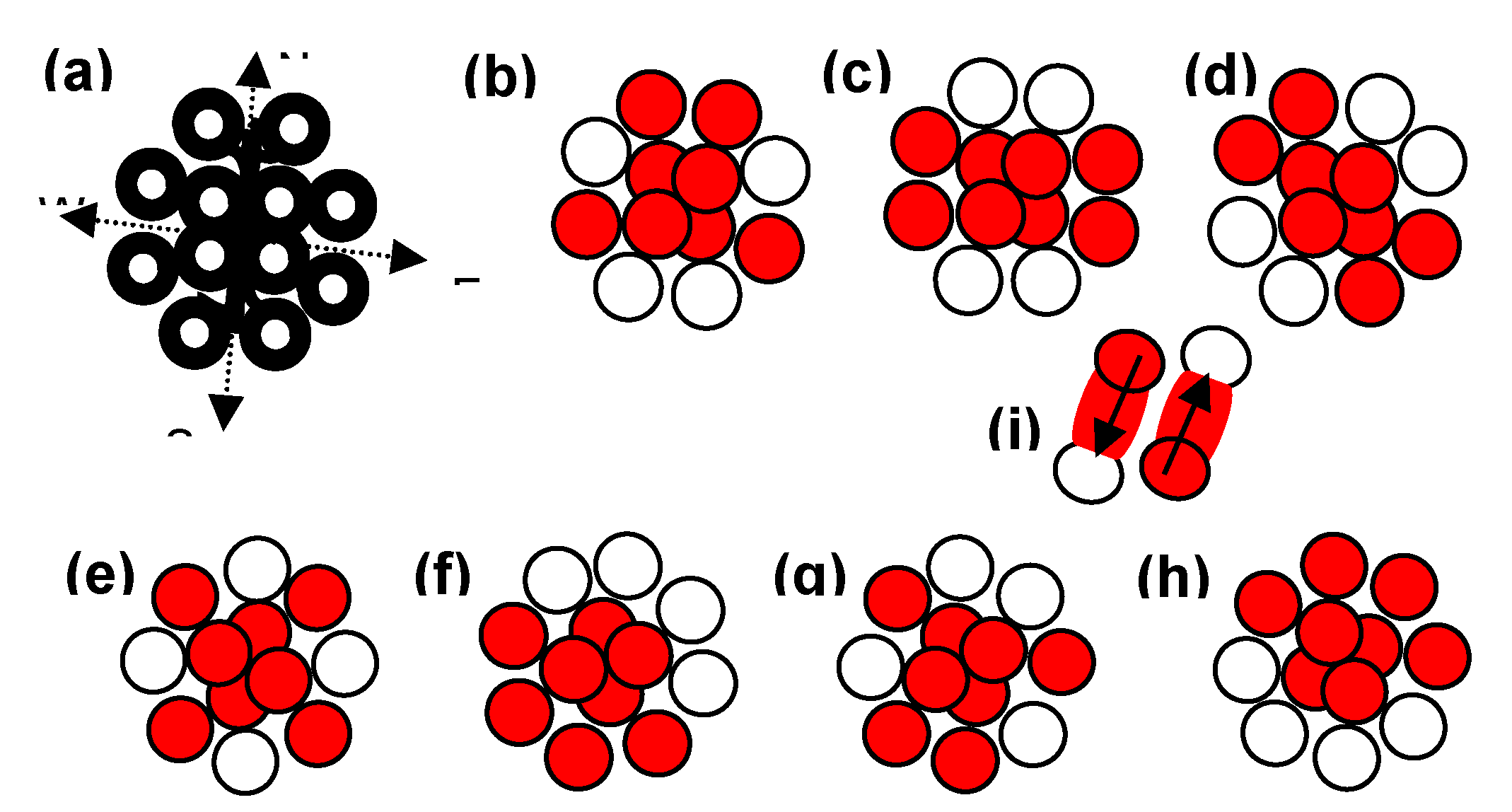
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




