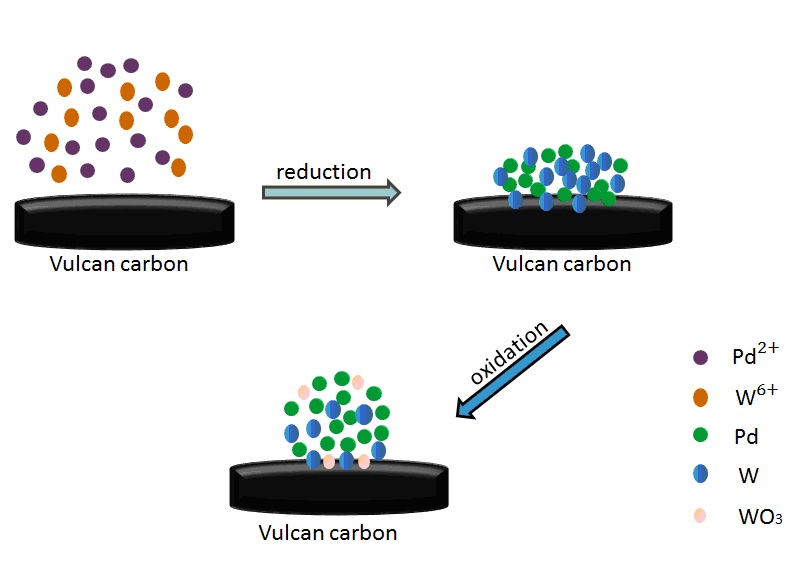
In this paper, we first report that WOx contained nanoalloys exhibit stable electrocatalytic performance in alkaline media, though bulk WO3 are easy to be dissolved in NaOH solutions. Carbon supported oxide-rich Pd-W alloy nanoparticles (PdW/C) with different Pd:W atom ratios were prepared by reduction-oxidation method. Among the catalysts, the oxide-rich Pd0.8W0.2/C (Pd/W = 8:2, atom ratio) exhibits the highest catalytic activity for oxygen reduction reaction. The X-ray photoelectron spectroscopy data shows that ~40% of Pd atoms and ~60% of the W atoms are in their oxides form. The Pd 3d5/2 peaks in oxide-rich Pd-W nanoalloys are positive shift compared with that of Pd/C, which indicates the electronic structure of Pd is affected by the strong interaction between Pd and W/WO3. Compare to Pd/C, the onset potential of oxygen reduction reaction at the oxide-rich Pd0.8W0.2/C is positive shifted. The current density (mA·mg Pd−1) at the oxide-rich Pd0.8W0.2/C is ~1.6 times of that at Pd/C. The oxide-rich Pd0.8W0.2/C also exhibits higher catalytic stability than Pd/C, which demonstrate that it is a prospective candidate for the cathode of fuel cells operated with alkaline electrolyte.