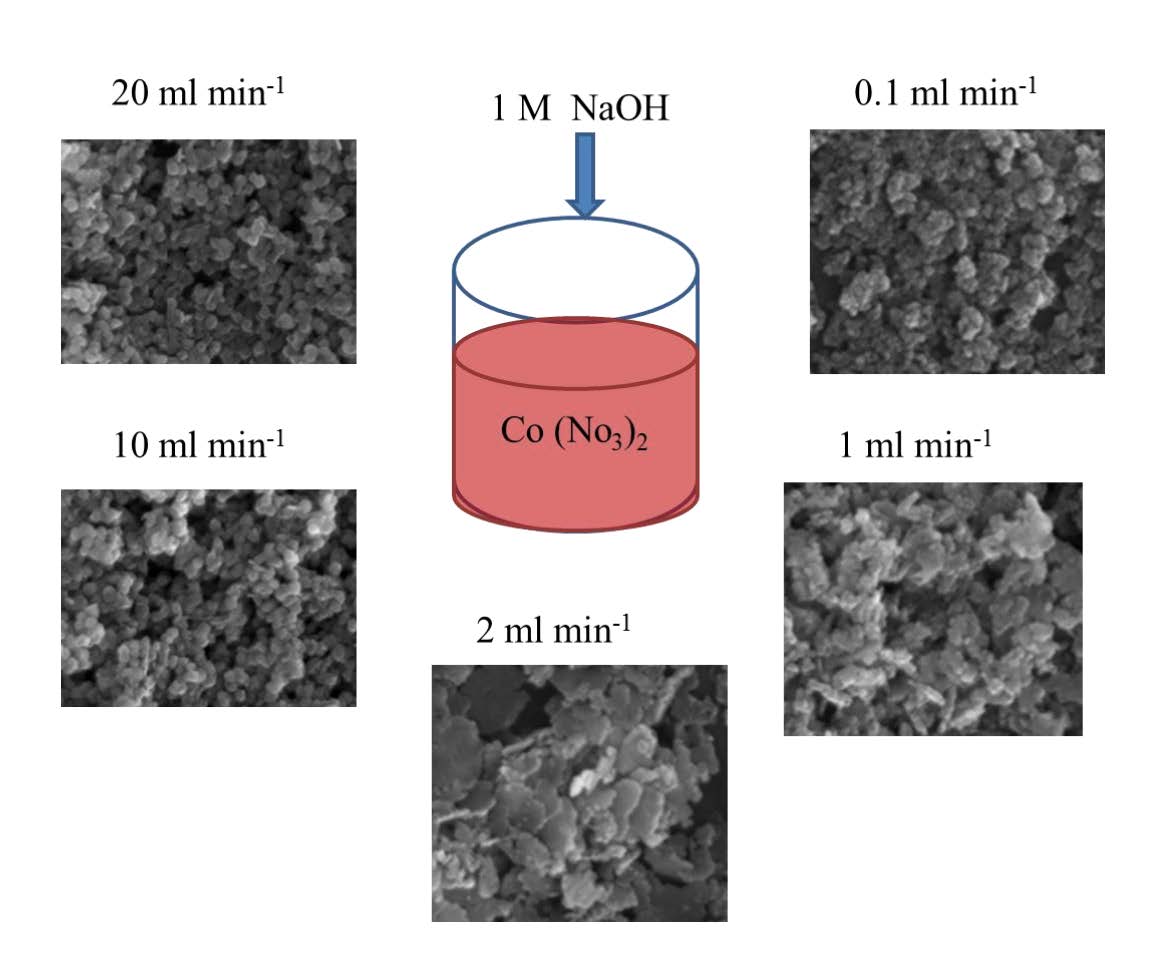
Crystalline β-cobalt hydroxide (β-Co(OH)2) of different morphologies have been successfully synthesized with the addition of sodium hydroxide to cobalt nitrate solution and aging in the mother liquor. The rate of NaOH addition, ranging from 0.1 mL/min to 10 mL/min, influences the surface morphology with the obtained storage capability of the respective electrode. Characterization of the β-Co(OH)2 was fully developed, including X-ray diffraction, scanning and transmission electron microscopy, and BET analyses. At a lower rate of NaOH addition, particles are like platelets, while for a higher rate (≥2 mL/min) grains are fused together forming a larger crystallite size. This result is supported by the X-ray diffraction structural analysis, where the phase evolution of (002) plane becomes distinct for the higher rate of NaOH addition. Lithium cobalt oxide (LiCoO2) was synthesized through oxidation from the as-prepared β-Co(OH)2 and LiOH. The electrochemical performance of as obtained LiCoO2 is investigated using charge-discharge and cyclic voltammetric studies. The precursor β-Co(OH)2 prepared at a lower rate of 0.1 mL/min in LiCoO2 demonstrated the best electrochemical performance of 155 mAh/g. After 50 cycles, the capacity retention rate was 67% compared with the first cycle. Finally, we have attempted to correlate the amount of the available OH- ions with the formation of platelets and the discharge capacity. This work has developed a methodology for the synthesis of LiCoO2 using β-Co(OH)2 in a facile chemical solution process.