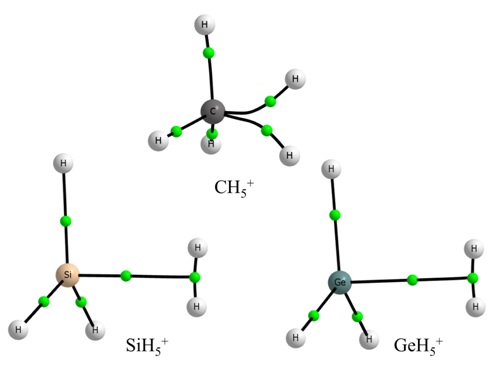
Atoms in Molecules (AIM), Natural Bond Orbital (NBO), and normal coordinate analysis have been carried out at the global minimum structures of TH5+ (T = C/Si/Ge). All these analyses lead to a consistent structure for these three protonated TH4 molecules. The CH5+ has a structure with three short and two long C-H covalent bonds and no H-H bond. Hence, the popular characterization of protonated methane as a weakly bound CH3+ and H2 is inconsistent with these results. However, SiH5+ and GeH5+ are both indeed a complex formed between TH3+ and H2 stabilized by a tetrel bond, with the H2 being the tetrel bond acceptor. The three-center-two-electron bond (3c-2e) in CH5+ has an open structure, which can be characterized as a V-type 3c-2e bond and that found in SiH5+ and GeH5+ is a T-type 3c-2e bond. This difference could be understood based on the typical C-H, Si-H, Ge-H and H-H bond energies. Moreover, this structural difference observed in TH5+ can explain the trend in proton affinity of TH4. Carbon is selective in forming a ‘tetrel bond’ and when it does, it might be worthwhile to highlight it as a ‘carbon bond’.