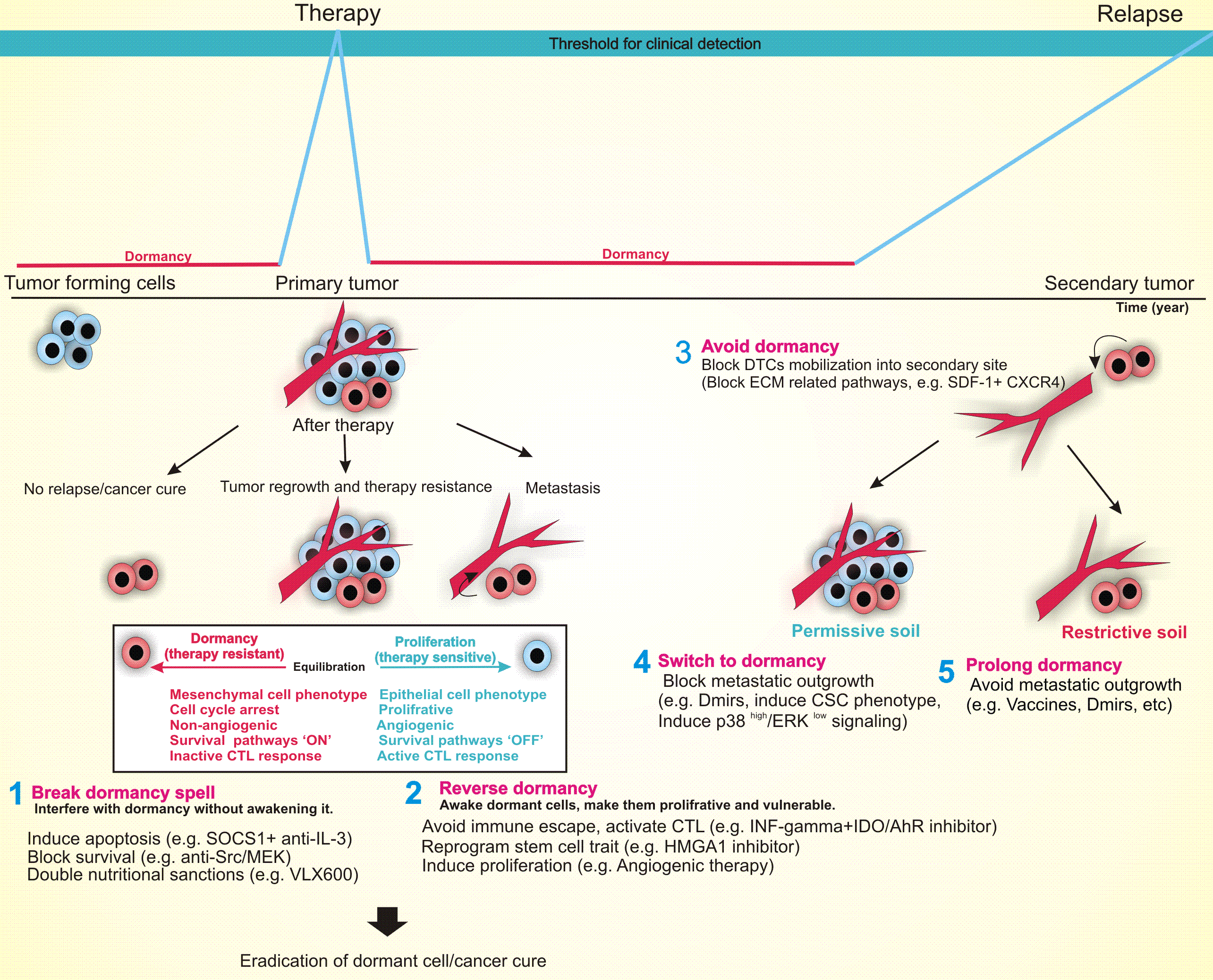
Tumor dormancy, a clinically undetectable state of cancer, makes a major contribution to the development of multidrug resistance (MDR), minimum residual disease (MRD), tumor outgrowth, cancer relapse, and metastasis. Despite its high incidence, the whole picture of dormancy-regulated molecular programs is far from clear. That is, it is unknown when and which dormant cells will resume proliferation causing late relapse, and which will remain asymptomatic and harmless to their hosts. Thus, identification of dormancy-related culprits and understanding their roles can help predict cancer prognosis and may increase the probability of a timely therapeutic intervention for the desired outcome. Here, we provide a comprehensive review of the dormancy-dictated molecular mechanisms, including an angiogenic switch, immune escape, cancer stem cells, extra cellular matrix (ECM) remodeling, metabolic reprogramming, miRNAs, epigenetic modifications, and stress-induced-p38 signaling pathways. Further, we analyze the possibility of leveraging these dormancy-related molecular cues to outmaneuver cancer, and discuss the implications of such approaches in cancer treatment.