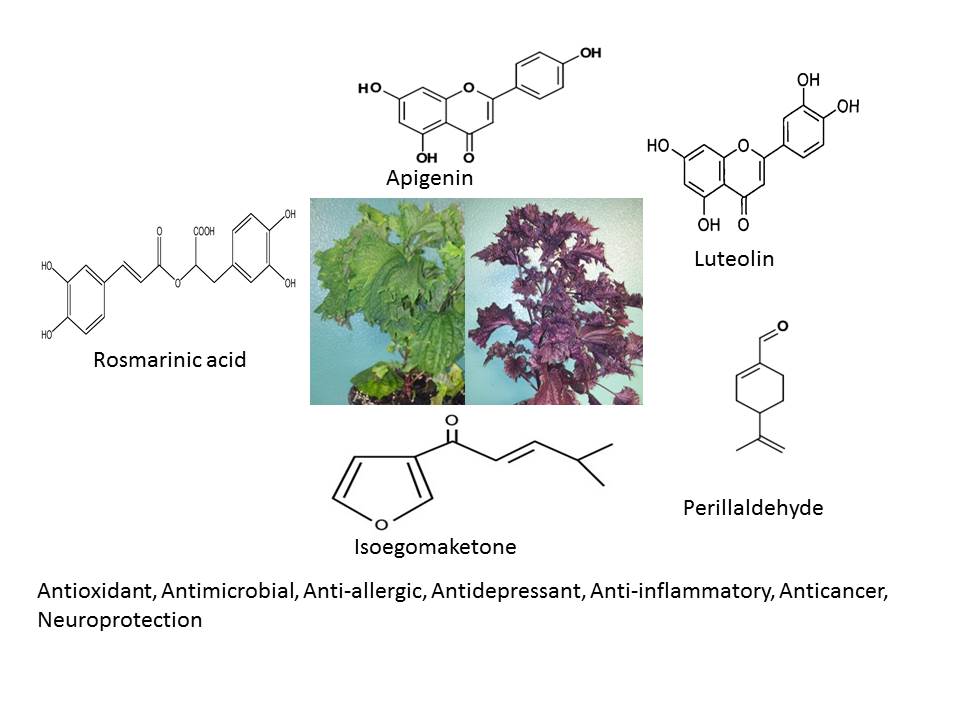
Perilla frutescens (L.) Britt. (PF) is an annual herbal medicinal, aromatic, functional food and ornamental plant that belongs to the mint family, Lamiaceae. The origin of perilla traces back to East Asian countries (China, Japan, Korea, Taiwan, Vietnam and India), where it has been used as a valuable source of culinary and traditional medicinal uses. Leaves, seeds and stems of P. frutescens are used for various therapeutic applications in folk medicine. In the absence of comprehensive review regarding all aspects of perilla, thus this review aims to present an overview pertaining to the botanical drug, ethnobotany, phytochemistry and biological activity. It was found that the taxonomic classification of perilla species is quite confused, and the number of species is vague. Perilla has traditionally been prescribed to treat depression-related disease, anxiety, asthma, chest stuffiness, vomiting, cough, cold, flus, phlegm, tumour, allergy, intoxication, fever, headache, stuffy nose, constipation, abdominal pain, indigestion, analgesic, anti-abortive agent, and sedative. Until now, 271 natural molecules have been identified in perilla organs including; polyphenols, flavonoids, essential oils, triterpenes, carotenoids, phytosterols, fatty acids, tocopherols and policosanols. In addition to solvent extracts, these individual compounds (rosmarinic acid, perillaldehyde, luteolin, apigenin, tormentic acid, isoegomaketone) have attracted researchers' interest for pharmacological properties. Its bioactivity showed antioxidant, antimicrobial, anti-allergic, antidepressant, anti-inflammatory, anticancer, neuroprotection activity. Although the results are promising in preclinical studies (in vitro and in vivo) as well, clinical studies are insufficient, therefore further study needs to be done to validate its therapeutic effects and to ensure its safety and efficacy.