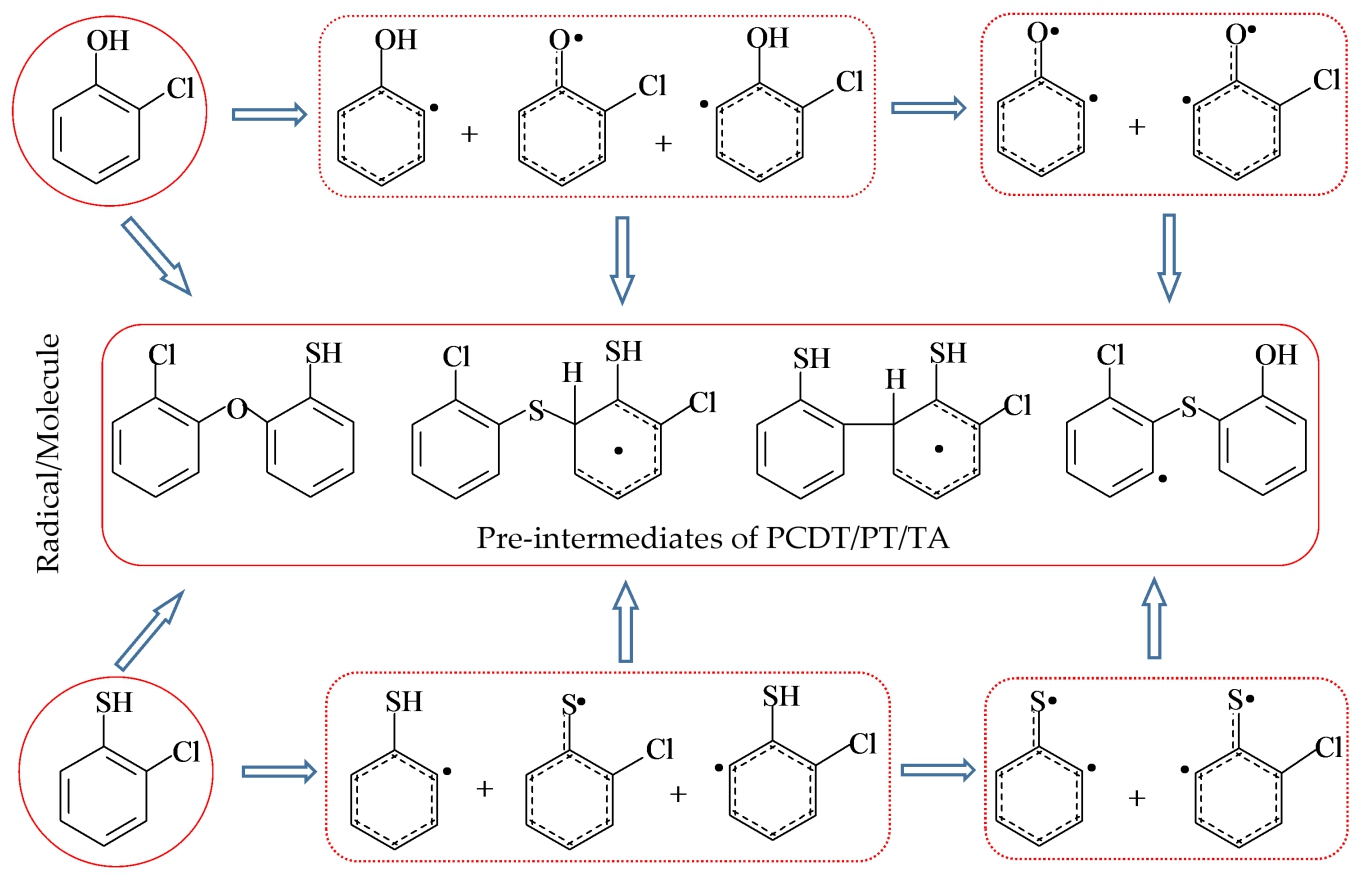
Polychlorinated phenoxathiins (PCPTs), polychlorinated dibenzothiophenes (PCDTs), and polychlorinated thianthrenes (PCTAs) are sulfur analogues of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/DFs). Chlorothiophenols(CTPs) and chlorophenols (CPs) are key precursors to form PCTA/PT/DTs, which can form chloro(thio)phenoxy radical, sulfydryl/hydryl-substituted phenyl radical and (thio)phenoxyl diradicals. The available radical/radical PCTA/DT formation mechanism failed to explain the higher concentration of PCDTs than that of PCTAs under the pyrolysis or combustion conditions. Thus in this work, a detailed thermodynamics and kinetic calculations were carried out to investigate the pre-intermediates formation for PCTA/PT/DTs from radial/molecule coupling of 2-C(T)P with their key radical species. Our study found that the radial/molecule mechanism can thermodynamically and kinetically contribute to the gas-phase formation of PCTA/PT/DT/s. The S/C coupling modes to form thioether-(thio)enol intermediats are preferable over the O/C coupling modes to form ether-(thio)enol intermediats. Thus, although the radial/molecule coupling of chlorophenoxy radical with 2-C(T)P have no effect on the PCDD/PTs formation, the radial/molecule coupling of chlorothiophenoxy radical with 2-C(T)P play an important role in the PCDT/PT formation. Most importantly, the pre-PCDT intermediates formation pathways from the coupling of sulfydryl/hydryl-substituted phenyl radical with 2-C(T)P and the coupling of (thio)phenoxyl diradicals with 2-C(T)P are more favorable to pre-PCTA/PT intermediates formation pathways from the coupling of chlorothiophenoxy radical with 2-C(T)P, which can give reasonable explanation for the high PCDT-to-PCTA ratio in the environment.