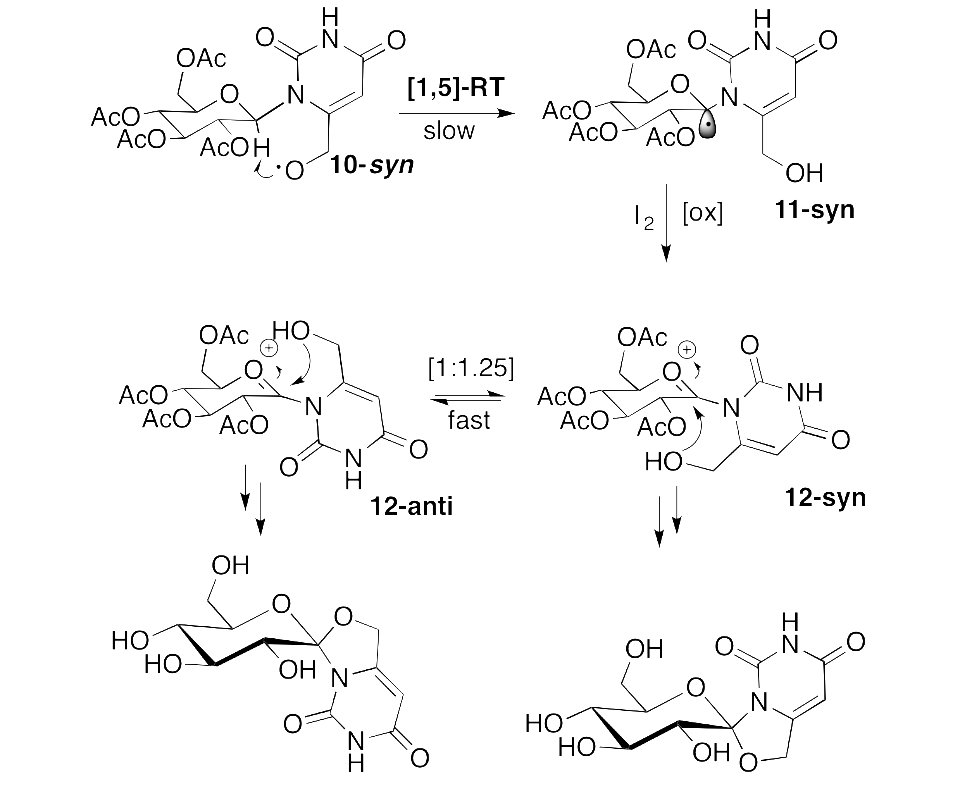
Glycogen phosphorylase (GP) has been a target for compounds, inhibitors of GP that might prevent unwanted glycogenolysis under high glucose conditions and thus aim at the reduction of excessive glucose production from the liver, in the case of type 2 diabetes. Anomeric spironucleosides, such as hydantocidin, present a rich synthetic chemistry and possess important biological function. We have successfully applied the Suárez radical methodology to synthesize the first example of a 1,6-dioxa-4-azaspiro[4.5]decane system, not been previously constructed via a radical pathway, starting from 6-hydroxymethyl-β-D-glucopyranosyluracil. We have shown that in the rigid pyranosyl conformation the required [1,5]-radical translocation is a minor process. The stereochemistry of the spirocycles obtained were unequivocally determined based on the chemical shifts of key sugar protons in the 1H NMR spectra. The two spirocycles were found to be modest inhibitors of RMGPb.