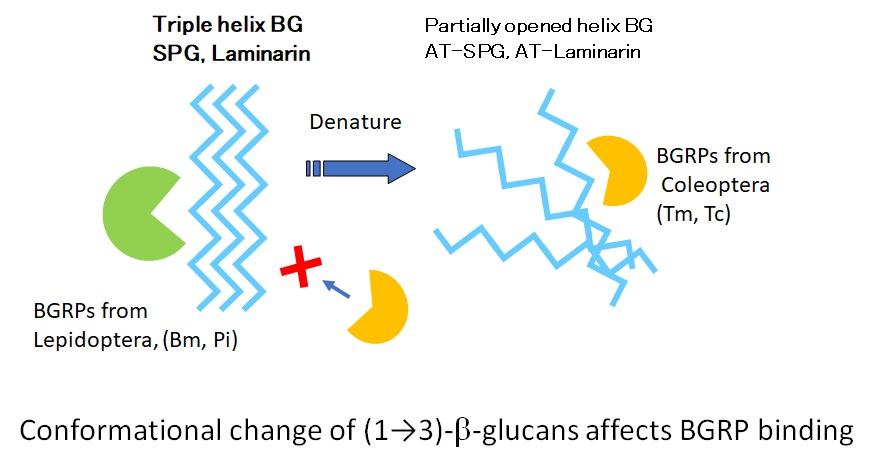
The recognition of (1→3)-β-D-glucans (BGs) by β-1,3-D-glucan recognition protein (BGRP) found in invertebrates plays a significant role in the activation of toll pathway and pro-phenol oxidase system in insect host defense against fungal invasion. To examine the structural diversity of BGs in BGRP interaction, the binding specificity of BGRPs cloned from four different insectswas characterized using ELISA. Recombinant BGRPs expressed as Fc-fusion proteins of human IgG1 bound to solid phase BGs. Because of the binding specificities, the BGRPs were categorized into two different ultrastructure- binding characters. The BGRPs from Silkworm and Indian meal moth bound to BGs containing triple-helical structure. Other BGRPs from red flour beetle and yellow mealworm beetle showed no binding to triple-helical BGs, but to alkaline-treated BGs, which have partially opened helical conformation. These evidences suggest that the innate immune system distinguishes different BG conformations and it is equipped for the diversity of BG structures.