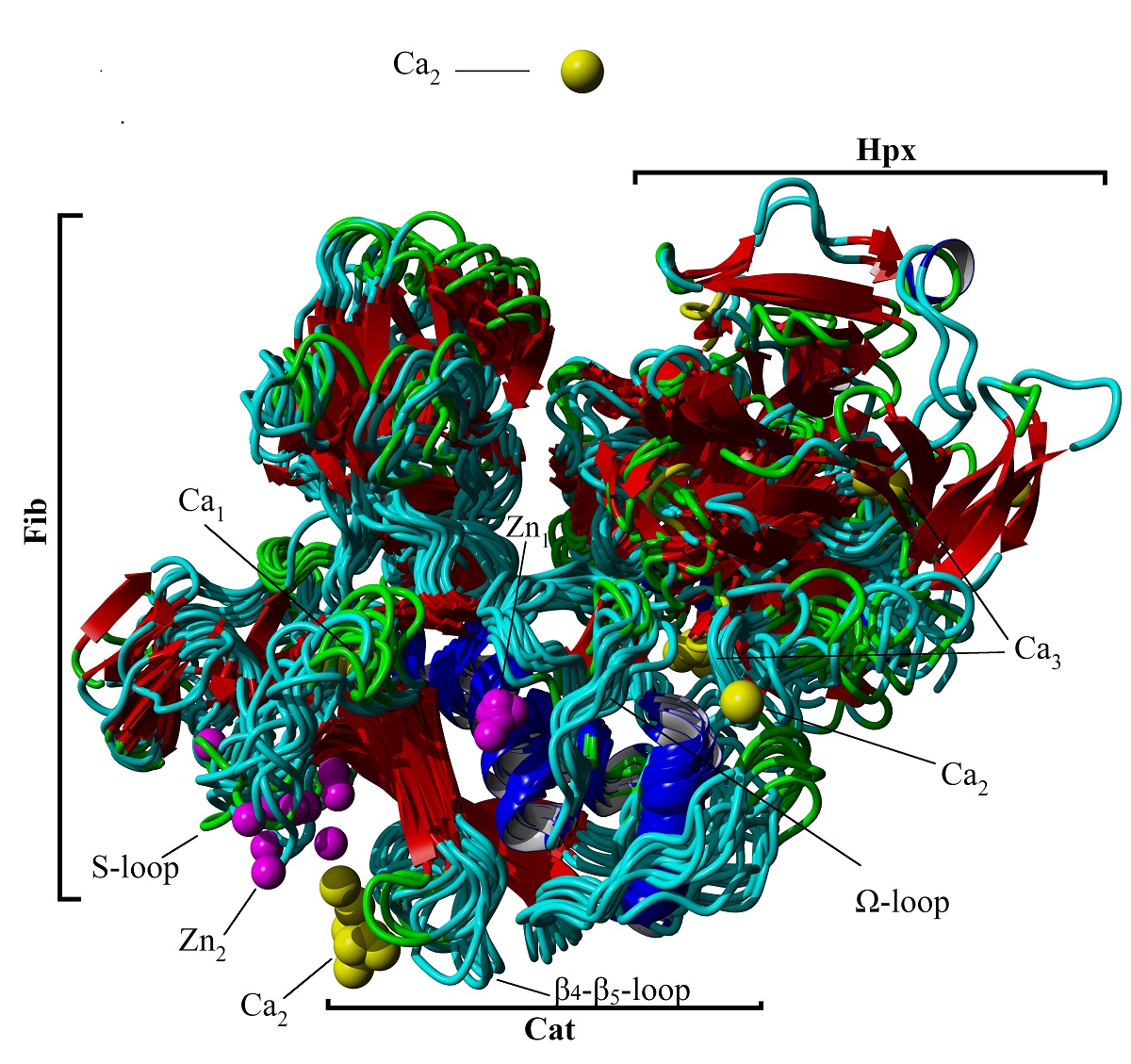
Matrix Metaloproteinase-2 (MMP-2) is an extracellular Zn2+ protease specific to type I and IV collagens. Its expression is associated with several inflammatory, degenerative, and malignant diseases. Conformational properties, domain movements, and interactions between MMP-2 and its associated metal ions were characterized using a 1.0 µs molecular dynamics simulation. Dihedral principle component analysis revealed 10 families of conformations with the greatest degree of variability occurring in the link region connecting the catalytic and hemopexin domains. Dynamics cross correlation analysis indicated domain movements corresponding to opening and closing of the hemopexin domain in relation to the fibronectin and catalytic domains facilitated by the link region. Interaction energies were calculated using the MMPBSA-interaction entropy analysis method and revealed strong binding energies for the catalytic Zn2+ ion 1, Ca2+ ion 1, and Ca2+ ion 3 with significant conformational stability at the binding sites of Zn2+ ion 1 and Ca2+ ion 1. Ca2+ ion 2 diffuses freely away from its crystallographically defined binding site. Zn2+ ion 2 plays a minor role in conformational stability of the catalytic domain while Ca2+ ion 3 is strongly attracted to the highly electronegative sidechains of the Asp residues around the central β-sheet core of the hemopexin domain.