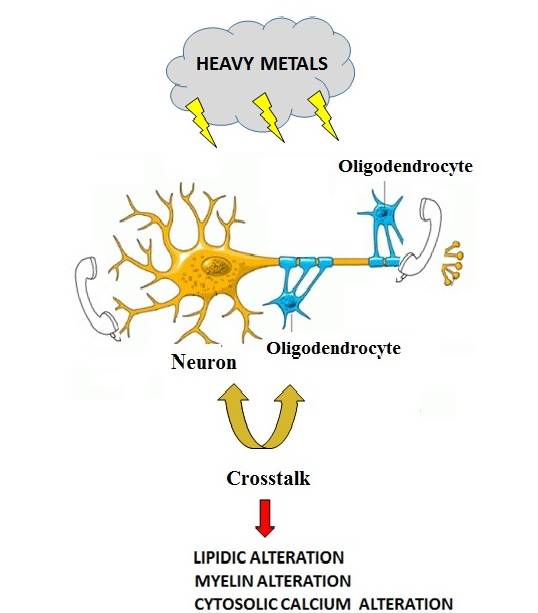
Evidence has been accumulated demonstrating that heavy metals may accumulate in various organs leading to tissue damage and toxic effects in mammals. In particular, the Central Nervous System (CNS) seems to be particularly vulnerable to cumulative concentrations of heavy metals, though the pathophysiological mechanisms is still to be clarified. In particular the potential role of oligodendrocyte dysfunction and myelin production after exposure to subtoxic concentration of heavy metals is to be better assessed. Here we investigated on the effect of sub-toxic concentration of several essential (Cu2 +, Cr3+, Ni2+, Co2+) and non-essential (Pb2+, Cd2+, Al3+) heavy metals on MO3.13 and SHSY5Y human oligodendrocyte and neuronal cell lines (grown individually or in co-culture). In particular, exposure of both cell lines to heavy metals produced a reduced cell viability of co-cultured cell lines compared to cells grown separately. This effect was more pronounced in neurons which were more sensitive to metals than oligodendrocytes when the cells were grown in co-culture. On the other hand, a significant reduction of lipid component in cells occurred after their exposure to heavy metals, an effect accompanied by substantial reduction of the main protein that makes up myelin (MBP) in co-cultured cells. Finally, the effect of heavy metals in oligodendrocytes were associated to imbalanced intracellular calcium ion concentration as measured through the fluorescent Rhod-2 probe, thus confirming that heavy metals, even used at subtoxic concentrations, lead to dysfunctional oligodendrocytes. In conclusion, our data show, for the first time, that sub-toxic concentrations of several heavy metals lead to dysfunctional oligodendrocytes, an effect highlighted when these cells are co-cultured with neurons. The pathophysiological mechanism(s) underlying this effect is to be better clarified. However, imbalanced intracellular calcium ion regulation, altered lipid formation and, finally, imbalanced myelin formation seem to play a major role in early stages of heavy metal-related oligodendrocyte dysfunction.