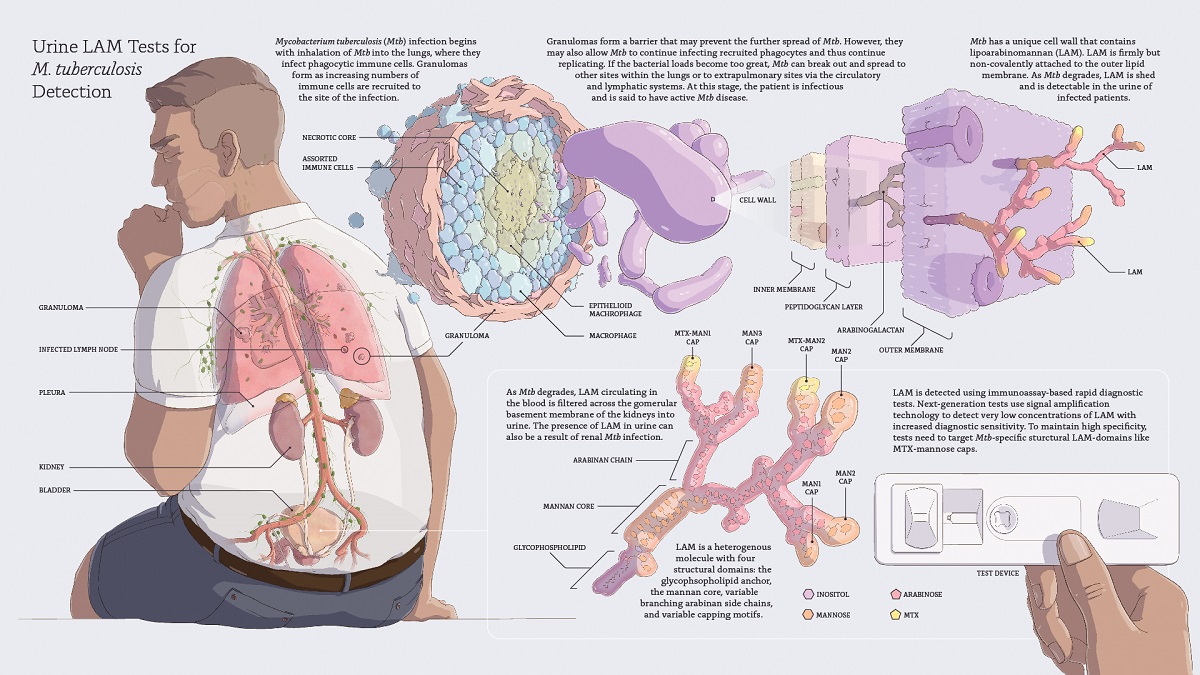
Most diagnostic tests for tuberculosis (TB) rely on sputum samples, which are difficult to obtain and have low sensitivity in immunocompromised patients, patients with disseminated TB, and children, delaying treatment initiation. The World Health Organization (WHO) calls for the development of a rapid, biomarker-based, non-sputum test capable of detecting all forms of TB at the point-of-care to enable immediate treatment initiation. Lipoarabinomannan (LAM) is the only WHO-endorsed TB biomarker which can be detected in urine, an easily collected sample. This review discusses the characteristics of LAM as a biomarker, describes the performance of first-generation urine LAM tests and reasons for slow uptake, and presents considerations for developing the next-generation of more sensitive and impactful tests. Next-generation urine LAM tests have the potential to reach adult and pediatric patients regardless of HIV status or site of infection and facilitate global TB control. Implementation and scale-up of existing LAM tests and development of next-generation assays should be prioritized.