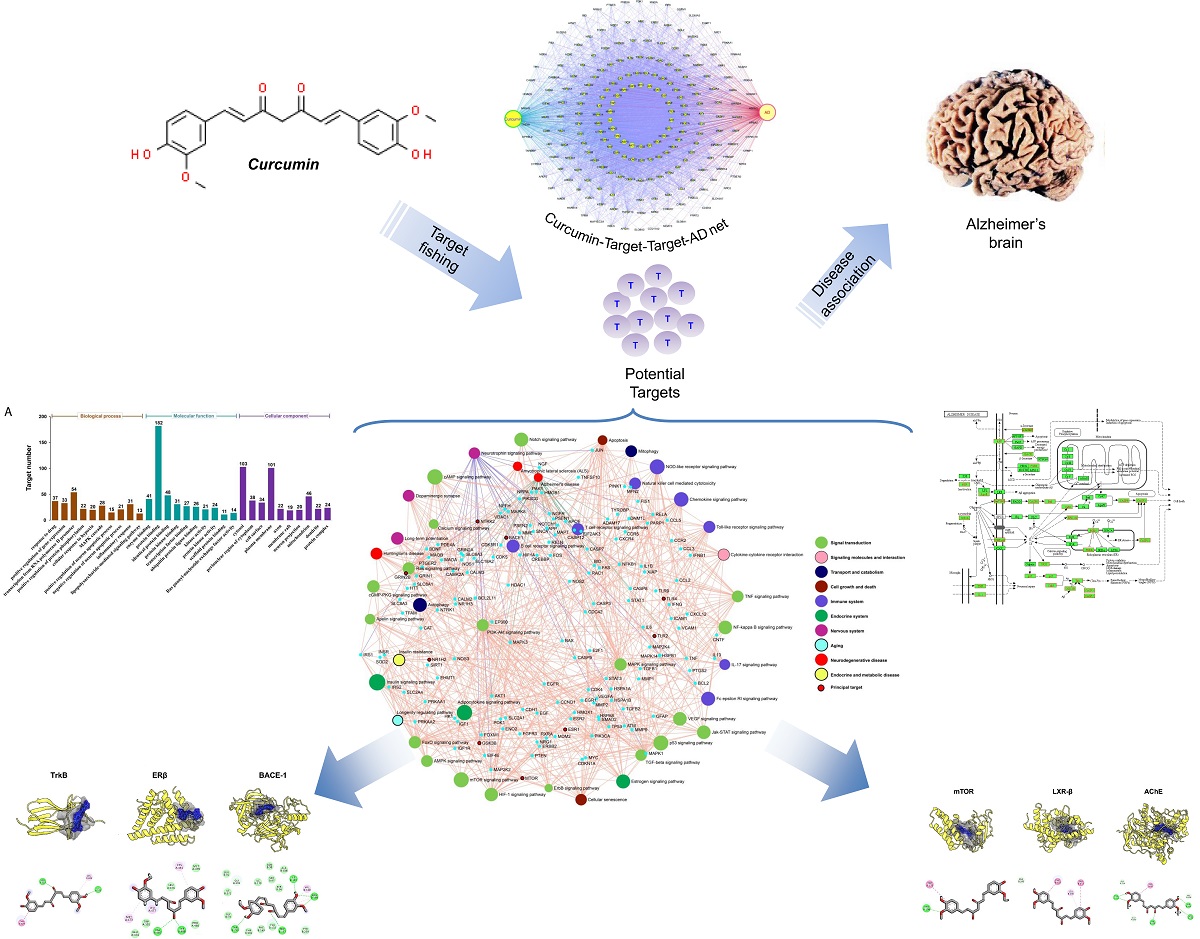
Curcumin is one of the bioactive metabolites of turmeric (Curcuma longa), known for its pleiotropic pharmacological actions, including antioxidant and anti-inflammation, anticholinesterase, immunomodulation, and neuroprotection. Substantial evidence suggests the therapeutic benefits of curcumin against neurodegenerative disorders, including Alzheimer’s disease (AD), acting on a diverse array of brain targets that make the molecular mechanisms complicated. System biology level-investigation could potentially present a comprehensive molecular mechanism to delineate the neuropharmacological action of curcumin. In this study, we used integrated system pharmacology and molecular simulation analysis to gain insights into the underlying mechanism of curcumin action against AD. Network pharmacology study identified curcumin-targeted potential cellular pathways such as phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling, neurotrophin signaling, toll-like receptor (TLR) signaling, and autophagy, and proteins such as tropomyosin receptor kinase B (TrkB), liver X-receptor-beta (LXR-β), estrogen receptor-β (ER-β), mammalian target of rapamycin (mTOR), TLR-2, N-methyl-D-acetate receptor subunit 2B (GluN2B), β-secretase and glycogen synthase kinase-3β (GSK-3β), which are intimately associated with neuronal growth and survival, immune response, and inflammation. Moreover, the molecular modeling further verified that curcumin showed a significant binding affinity to mTOR, TrkB, LXR-β, TLR-2, ER-β, GluN2B, β-secretase, and GSK-3β, which are the crucial regulators of molecular and cellular processes associated with AD. Together, the present system pharmacology and in silico findings demonstrate that curcumin might play a significant role in modulating AD-pathobiology, supporting its therapeutic application for the prevention and treatment of AD.