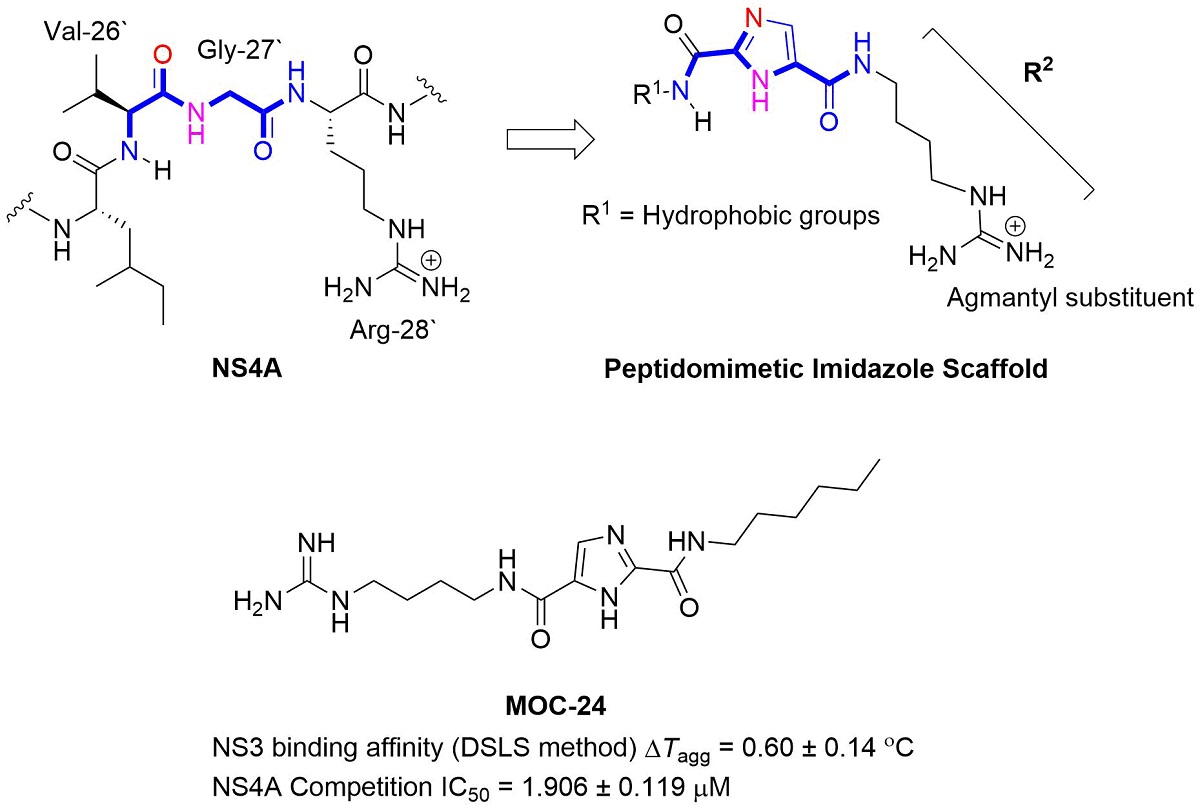
The non-structural protein NS3/4A protease is a critical factor for hepatitis C virus (HCV) maturation that requires activation by NS4A. Synthetic peptide mutants of NS4A were found to inhibit NS3 function. The bridging from peptide inhibitors to heterocyclic peptidomimetics of NS4A has not been in consideration in literature, and therefore, we decided to explore this strategy to develop a new class of NS3 inhibitors. In this report, a structure-based design approach was used to convert the bound form of NS4A into 1H-imidazole-2,5-dicarboxamide derivatives as first generation peptidomimetics. This scaffold mimics the buried amino acid sequence Ile-25` to Arg-28` at the core of NS4A21`-33` needed to activate the NS3 protease. Some of the synthesized MOC compounds were able to compete with and displace NS4A21`-33` for binding to NS3. For instance, N5-(4-guanidinobutyl)-N2-(n-hexyl)-1H-imidazole-2,5-dicarboxamide (MOC-24) inhibited the binding of NS4A21`-33` with a competition IC50 of 1.9 ± 0.12 µM in a fluorescence anisotropy assay, stabilized the denaturation of NS3 by increasing the aggregation temperature by ΔTagg 0.6 ± 0.140 ℃. MOC-24 also inhibited NS3 protease activity in a fluorometric assay. Molecular dynamics simulations rationalized the structure-activity relationship (SAR) differences between the active MOC-24 and the inactive MOC-26. Our data shows that MOC compounds are possibly the first examples of NS4A peptidomimetics that demonstrated promising activities against NS3 proteins.