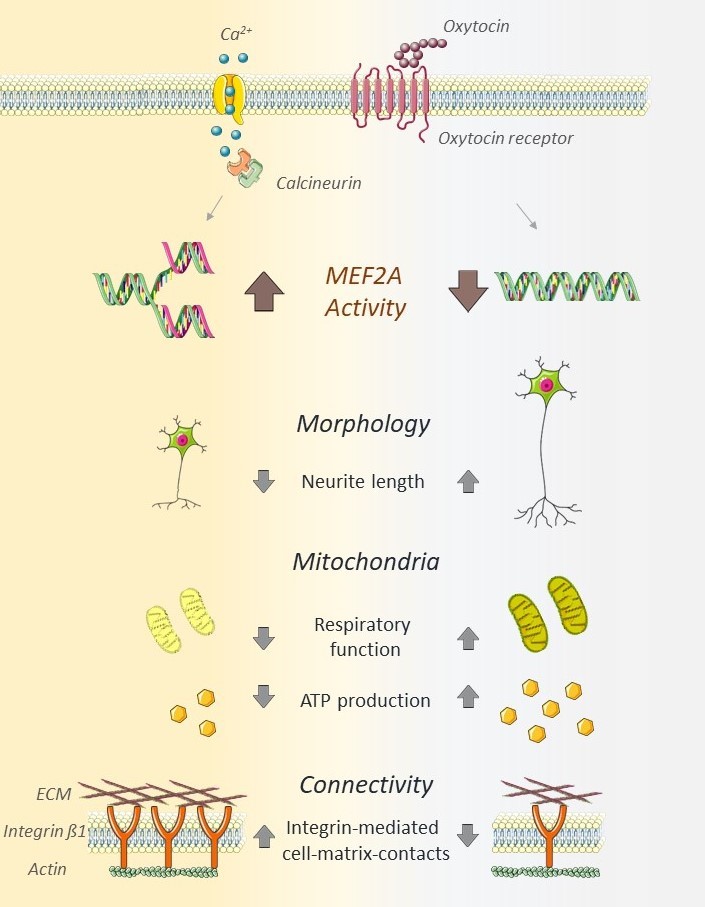
The neuropeptide oxytocin (OT) is a well-described modulator of socio-emotional traits, such as anxiety, stress, social behavior, and pair-bonding, however, when dysregulated, it is associated with adverse psychiatric traits, like various aspects of autism spectrum disorder (ASD). In this study, we identify the transcription factor MEF2A as the common link between OT and cellular changes symptomatic for ASD, encompassing neuronal morphology, connectivity, and mitochondrial function. We provide evidence for MEF2A as the decisive factor defining the cellular response to OT: while OT induces neurite retraction in MEF2A expressing neurons, OT causes neurite outgrowth in absence of MEF2A. A CRISPR-Cas-mediated knockout of MEF2A and retransfection of an active version or permanently inactive mutant, respectively, validated our findings. We also identified the phosphatase calcineurin as the main upstream regulator of OT-induced MEF2A signaling. Further, MEF2A signaling dampens mitochondrial functioning in neurons, as MEF2A knockout cells show increased maximal cellular respiration, spare-respiratory capacity, and total cellular ATP. In summary, we reveal a central role for OT-induced MEF2A as major regulator of cellular morphology as well as neuronal connectivity and mitochondrial functioning, with broad implications for a potential treatment of disorders based on morphological alterations or mitochondrial dysfunction.