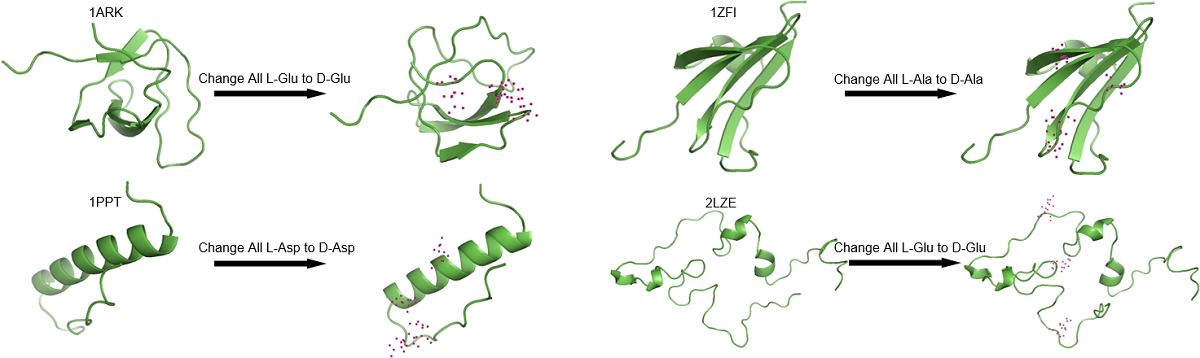
On the primitive Earth, both L- and D-amino acids would have been present. However, only L-amino acids are essential blocks to construct proteins in modern life. To study the relative stability of homochiral and heterochiral peptides, a variety of computational methods were employed. 10 prebiotic amino acids (Gly, Ala, Asp, Glu, Ile, Leu, Pro, Ser, Thr, and Val) were previously determined by multiple previous meteorite, spark discharge, and hydrothermal vent studies. We focused on what had been reported as primary early Earth polypeptide analogs: 1ARK, 1PPT, 1ZFI, and 2LZE. Tripeptide composed of only Asp, Ser, and Val exemplified that different positions (i.e., N-terminus, C-terminus, and middle) made a difference in minimal folding energy of peptides, while the classification of amino acid (hydrophobic, acidic, or hydroxylic) did not show significant difference. Hierarchical cluster analysis for dipeptides with all possible combinations of the proposed 10 prebiotic amino acids and their D-amino acid substituted derivatives generated five clusters. Prebiotic polypeptides were built up to test the significance of molecular fluctuations, secondary structure occupancies, and folding energy differences based on these clusters. Most interestingly, among 129 residues, mutation sensitivity profiles presented that the ratio of more stable to less stable to equally stable D-amino acids was about 1:1:1. In conclusion, some combinations of a mixture of L- and D-amino acids can act as essential building blocks of life. Peptides with α-helices, long β-sheets, and long loops are usually less sensitive to D-amino acid replacements in comparison to short β-sheets.