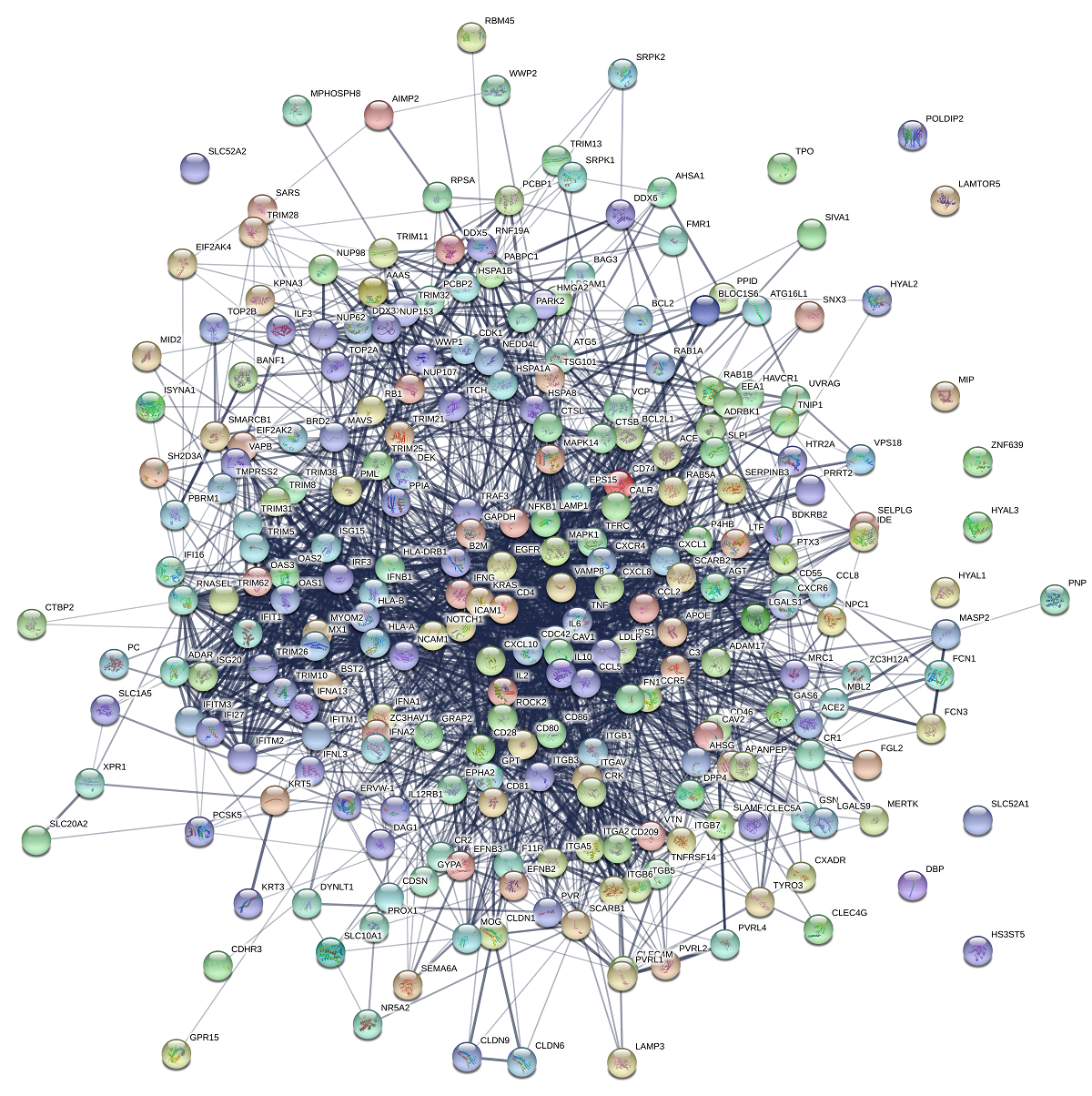
COVID-19 (2019-nCoV) is a pandemic disease with an estimated mortality rate of 3.4% (estimated by the WHO as of March 3, 2020). Until now there is no antiviral drug and vaccine for COVID-19. The current overwhelming situation by COVID-19 patients in hospitals is likely to increase in the next few months. About 15 percent of patients with serious disease in COVID-19 require immediate health services. Rather than waiting for new anti-viral drugs or vaccines that take a few months to years to develop and test, several researchers and public health agencies are attempting to repurpose medicines that are already approved for another similar disease and have proved to be fairly effective. This study aims to identify FDA approved drugs that can be used for drug repurposing and identify biomarkers among high- risk and asymptomatic groups. In this study gene-disease association related to COVID-19 reported mild, severe symptoms and clinical outcomes were determined. The high-risk group was studied related to SARS-CoV-2 viral entry and life cycle by using Disgenet and compared with curated COVID-19 gene data sets from the CTD database. The overlapped gene sets were enriched and the selected genes were constructed for protein-protein interaction networks. Through interactome, key genes were identified for COVID-19 and also for high risk and asymptomatic groups. The key hub genes involved in COVID-19 were VEGFA, TNF, IL-6, CXCL8, IL10, CCL2, IL1B, TLR4, ICAM1, MMP9. The identified key genes were used for drug-gene interaction for drug repurposing. The chloroquine, lenalidomide, pentoxifylline, thalidome, sorafenib, pacitaxel, rapamycin, cortisol, statins were proposed to be probable drug repurposing candidates for the treatment of COVID-19. However, these predicted drug candidates need to be validated through randomized clinical trials. Also, a key gene involved in high risk and the asymptomatic group were identified, which can be used as probable biomarkers for early identification.