1. Introduction
Why did inanimate materials evolve into complex organisms and societies on Earth? This is a fundamental question in science that has captivated humans for millennia [
1]. Creationists claim that humans, life, Earth, and the whole universe were created by one or more supernatural powers [
2]. Ancient Greek philosophers proposed various hypotheses to explain the evolution. For example, Anaximander posited that apeiron, which is a substance without fixed boundaries and forms, gives rise to opposites such as cold and hot, dry and wet, and these opposites, in turn, produce all things, including organisms [
1].
Historically, the notion of spontaneous generation held that some organisms could arise spontaneously from non-living matter, such as fleas, which could arise from decaying matter [
1]. This notion was widely accepted before the 18th century.
In physics, the notion of negative entropy proposed by Nobel laureate Erwin Schrödinger and the dissipative structure theory proposed by Nobel laureate Ilya Prigogine employed various abstract concepts and mathematical equations to interpret the origin of the orderliness of organisms [
3,
4]. These notions or theories did not provide direct or explicit answers about evolution [
3,
4], and their validity has been challenged [
1,
5].
In chemistry, in the 1920s, Alexander Oparin proposed a hypothesis suggesting that life on Earth originated through a gradual chemical evolution of organic molecules [
6]. Since the 1950s, many experiments have been conducted to investigate how various organic molecules, such as amino acids, monosaccharides, nucleotides, proteins, and nucleic acids, could be synthesized naturally on the prebiotic Earth [
7,
8,
9,
10,
11,
12,
13,
14,
15]. The concept of chemical evolution has been widely accepted, although the processes involved have not been fully revealed. In the 1970s, the hypercycle theory proposed by Nobel laureate Manfred Eigen assumed that some autocatalytic or self-replicating macromolecules are interconnected in a way that each of them catalyzes the production of its successor, with the last molecule catalyzing the first one [
16]. The hypercycles could reinforce themselves and extend with the incorporation of new molecules under a natural selection process [
16].
In biology, various evolutionary theories have been proposed, including the natural selection hypothesis proposed by Charles Darwin in the 19th century and its updated version, the Modern Synthesis, which emerged in the 20th century [
17]. Darwin's theory elucidated the importance of natural selection, and the Modern Synthesis elucidated the genetic basis underlying it [
17]. These two theories cannot explain some macroevolution issues, such as the origin of life and multicellular organisms, because organisms are no fitter than inanimate materials, and multicellular organisms are no fitter than unicellular organisms [
17]. Additionally, Darwin's theory and the Modern Synthesis clarified the existence of competition and natural selection of organisms, but they did not clarify why organisms can exist.
In the social sciences, most social evolutionary theories in the 19th century claimed that human societies evolved from a primitive state to a more civilized one along a unilineal path. In the 20th century and recent years, social evolutionary theories have mainly focused on the changes specific to individual human societies (multilineal evolution) [
18,
19,
20]. Some social evolution theories, inspired by Darwin's theory, led to notorious notions, such as social Darwinism, which highlighted fierce competition and justified the policies of colonialism, slavery, racism, and massacre [
18,
19,
20]. These theories have not revealed the natural roots of some key social notions, such as inclusiveness, altruism, and collaboration.
In general, previous evolutionary theories have been shown to be incorrect (e.g., spontaneous generation), indirect (e.g., some theories in physics), or incomplete (e.g., Darwinism, the Modern Synthesis, and some social evolutionary theories). Furthermore, they did not unify the origin of life, biological evolution, and social evolution in a direct and explicit way. Therefore, it is desirable to engage in theoretical research that addresses these evolutionary questions.
2. Definitions
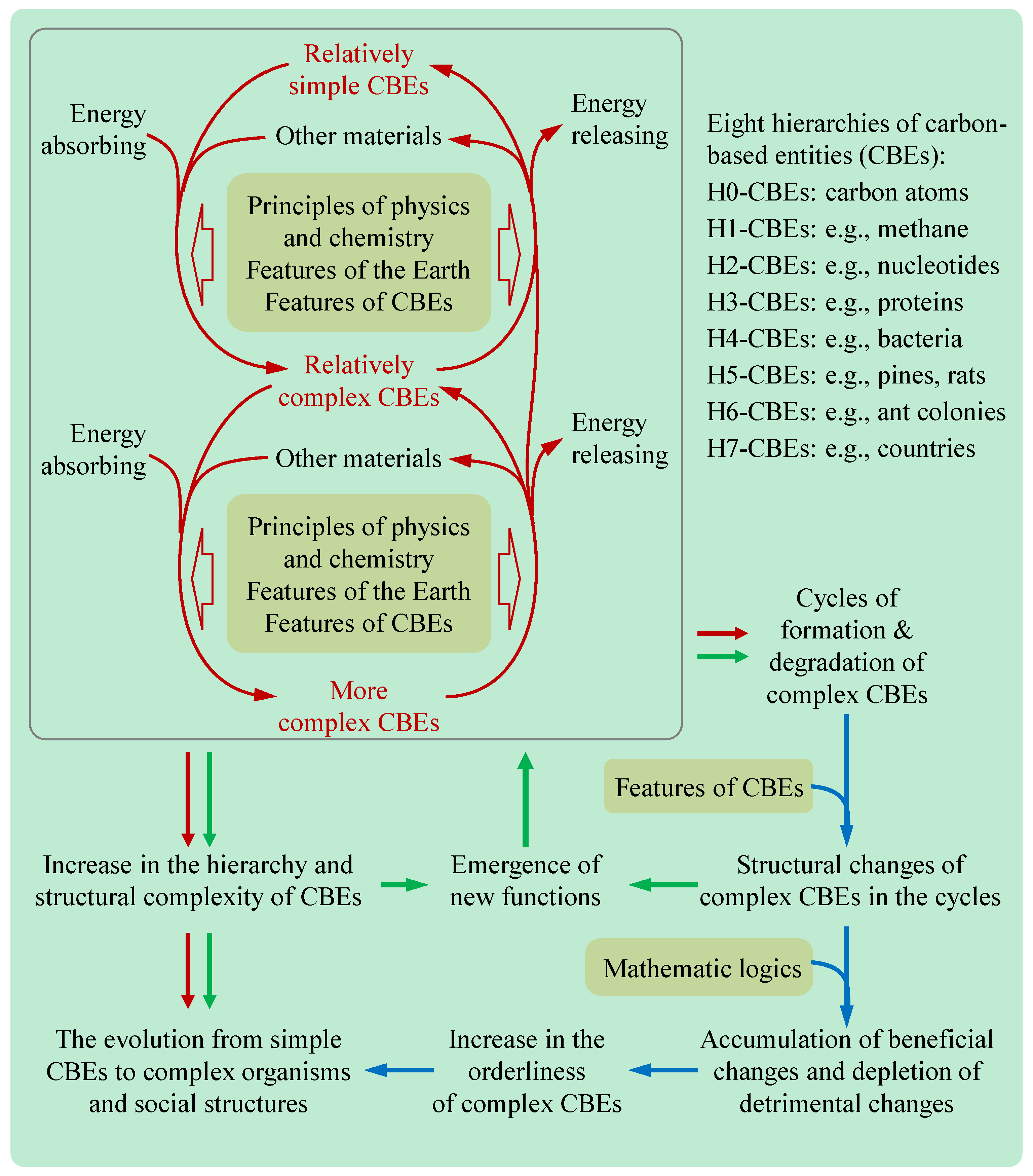
Here we classify carbon-based entities (CBEs) into eight hierarchies (H0−H7) (
Table 1). H0-CBEs refer to carbon atoms. H1-CBEs refer to small carbon-containing molecules (CCMs), such as methane and carbon dioxide. H2-CBEs refer to intermediate CCMs, such as lysine and glucose. H3-CBEs refer to large CCMs, such as proteins and nucleic acids, composed of H2-CBE residues and some functional groups. H4-CBEs refer to cells, such as bacteria, composed of various H3-CBEs and other molecules. H5-CBEs refer to multicellular organisms, such as pines and rabbits, composed of some H4-CBEs (cells) and other materials. H6-CBEs refer to animal social collectives, such as ant colonies and bee colonies, composed of some animal individuals that closely collaborate for the collective good and have distinct roles within the collectives. H6-CBEs have only one hierarchy in management. H7-CBEs refer to human social collectives that have multiple hierarchies in management, and low-hierarchy collectives closely collaborate with different duties for high-hierarchy collectives. For example, a university is an H7-CBE as it possesses the hierarchies of departments, colleges, and the whole university in management. Also, an army and a country are H7-CBEs as they have multiple hierarchies in management.
Notably, no clear lines separate these CBE hierarchies. For example, some peptides are between H2-CBEs and H3-CBEs, viruses are between H3-CBEs and H4-CBEs, and lion groups are between H5-CBEs and H6-CBEs.
High-hierarchy CBEs (HHCBEs) and low-hierarchy CBEs (LHCBEs) are defined by comparing their hierarchies. For example, H4-CBEs are HHCBEs compared to H3-CBEs, but they are LHCBEs compared to H5-CBEs.
Simple CBEs and complex CBEs are defined by comparing their structural complexity. For example, eukaryotic paramecia are complex CBEs compared to prokaryotic staphylococci, but they are simple CBEs compared with multi-cellular ants.
Sometimes LHCBEs or simple CBEs refer to H0-CBEs to H3-CBEs, while HHCBEs or complex CBEs refer to H4-CBEs to H7-CBEs.
We define the entire evolution process from H1-CBEs to H7-CBEs as the carbon-based evolutionary process (CBEP), and define the related evolutionary theory as the Carbon-Based Evolutionary Theory (CBET). The major viewpoints of the CBET are given in
Figure 1.
Table 2.
Core viewpoints of the Carbon-Based Evolutionary Theory (CBET).
Table 2.
Core viewpoints of the Carbon-Based Evolutionary Theory (CBET).
| Core viewpoints |
Explanations |
| 1. The energy dissipated from the permanent energy sources, such as sunlight, geothermal energy, cosmic radiation, water flow, wind, etc., is adjusted by the atmosphere and the abundant water on Earth. Many substances on Earth spontaneously or actively absorb energy from these sources under some well-known principles of physics and chemistry (e.g., laws of thermodynamics). This constitutes the driving force mechanism that provides energy for the evolution of CBEs on Earth. Some relative simple CBEs can form relatively complex CBEs due to their special features after energy absorption. |
Energy is essential for the synthesis of organic molecules, the growth of plants, the movement of animals, the reproduction of organisms, and the development of human society. Among all atoms, only carbon can lead other atoms to form multiple hierarchies of structures due to some features of carbon atoms and other CBEs. Among all known planets, only Earth has been found to support life due to its rare habitable features. These features that have been overlooked in previous evolutionary theories are essential to directly and explicitly explain the CBE evolution. |
| 2. Some complex CBEs formed through the above energy-absorption processes possess some new functions that less complex CBEs do not, and some structural variations of complex CBEs, which occur in the cycles of formation and degradation of complex CBEs as elucidated below, can change the functions of the complex CBE. These two facts constitute the structural mechanism that generates new functions for the evolution of CBEs on Earth.. |
This mechanism stems from the logic that a new structure can engender new functions. This mechanism is aided by the driving force mechanism and reinforced by the natural selection mechanism. |
| 3. The above two mechanisms lead to the formation and accumulation of various complex CBEs on Earth. |
The driving force mechanism and the structural mechanism are obvious in chemical evolution and biological evolution, respectively. |
| 4. Almost all complex CBEs will degrade, and complex CBEs regenerating due to the above two mechanisms usually carry structural variations due to some features of CBEs. Therefore, there are cycles of formation and degradation of complex CBEs with structural variations in complex CBEs. 5. The cycles, in mathematics, lead to the accumulation of the variations beneficial to the formation and maintenance of complex CBEs and the depletion of detrimental variations, which constitutes the natural selection mechanism. |
Natural selection was explained by previous theories with the phenomenon of survival competition in organisms. Natural selection is explained in the CBET using its mathematical essence. The CBET also extends natural selection from biological evolution to the competition among organic molecules (chemical evolution) and the competition of animal and human social collectives (social evolution). |
| 6. The synergistic action of the above three mechanisms results in the progression from chemical to biological and social evolution, marked by the escalating hierarchy of CBEs and the increase in the quantity, diversity, and orderliness of high-hierarchy CBEs. |
The driving force mechanism and the structural mechanism explain why complex CBEs emerge on Earth and the natural selection explain why some complex CBEs cannot exist on Earth. |
3. Special Features of Earth and CBEs
3.1. Special Features of Earth
Feature 1: Earth has abundant energy sources, such as cosmic radiation, sunlight, lightning, geothermal energy, volcanoes, fires, water flow, wind, and the degradation of organic materials [
21].
Feature 2: Earth has abundant water, which weighs around 1.35×1018 tons [
22].
The energy released from the energy sources on Earth is regulated by the atmosphere, which is over 1,000 kilometers thick, and the abundant water on Earth. This regulation helps to maintain a moderate, widespread, and persistent distribution of energy on Earth and renders Earth an exceptionally rare and hospitable celestial body in astronomy [
21].
Water and energy are essential to the synthesis of organic molecules, the growth of plants, the movement of animals, the reproduction of organisms, and the development of human society. Besides adjusting the energy on Earth, water participates in the formation of all hierarchies of CBEs as an important constituent component and the environment of the formation. Water is also important to maintain the structures and functions of many CBEs. Given the crucial role of water and energy, tropical forests boast a greater abundance and variety of organisms compared to tropical deserts or cold regions.
3.2. Special Features of H0-CBEs (Carbon Atoms)
The following specific features of carbon atoms significantly contributed to the CBEP, which helps to explain why, among all substances on Earth, only CBEs have evolved from simple structures to highly complex structures.
Feature 1: Carbon is abundant on Earth. It has been estimated that the atmosphere, water bodies, and biosphere on Earth contain 9×1011, 36×1011, and 5.5×1011 tons of carbon, respectively [
23,
24]. There is also abundant carbon in stones, coals, petroleum, natural gas, and methane hydrates on Earth.
Feature 2: Carbon atoms can form chemical bonds with a wide range of other atoms and numerous intermediate CCMs that are soluble in water or oil. This is because carbon atoms are smaller than the other atoms (silicon, germanium, tin, and lead) that have four electrons in their valence shells [
23,
24].
Feature 3: Among all elements, carbon is unique in its ability to form long, relatively stable chains of interconnected bonds, which serve as the backbone for numerous large CCMs that are soluble in water. These backbones can bond with atoms such as hydrogen, oxygen, nitrogen, sulfur, phosphorus, halogens, metals, and various groups of atoms known as functional groups. The length, shape, and chirality of the chains all play a role in determining the properties of these large CCMs. No other atoms, including boron and silicon, are capable of forming the backbones of chains in large molecules that are as long, complex, or stable as carbon-based large organic molecules in water [
23,
24]. Because stable, complex, and water-soluble large molecules are essential for the construction of organisms, it is widely accepted that only CBEs have the potential to evolve from simple structures into life [
23,
24].
3.3. Special Features of Other CBEs (H1-CBEs to H7-CBEs)
Feature 4: Many CBEs are relatively stable on Earth. For example, some proteins have been well preserved in fossils for millions of years [
25], and various H2-CBEs have been preserved longer than proteins in meteorites [
26].
Feature 5: Almost all complex CBEs are degradable. Typically, an external factor such as fire, collision, or radiation can degrade a complex CBE by disrupting its component interactions. High temperatures, such as those from mountain fires, often accelerate the degradation of complex CBEs [
17]. Usually, it is easier to destroy a complex CBE than to generate it. For example, it takes only minutes with electricity to kill a pig that has been nourished for months. Additionally, HHCBEs cannot be directly formed from CBEs that are two or more hierarchies lower, while HHCBEs can degrade or be degraded into CBEs that are two or more hierarchies lower. For example, in anoxic and high-temperature environments, a living grass plant can be directly decomposed into carbon atoms (
Figure 1).
Feature 6: Many CBEs, along with other materials, can form more complex CBEs after energy absorption, and this will be elaborated in
Section 4.
Feature 7: Organic synthesis reactions of many H2-CBEs and H3-CBEs are complex, with products varying based on different starting materials and reaction conditions. Even when using the same starting material under identical reaction conditions, the products of these reactions are usually diverse. Furthermore, HHCBEs, ranging from H4-CBEs to H7-CBEs, are multifarious because they result from the recombination of various LHCBEs.
4. The Driving Force Mechanism
As shown in
Section 2, Earth possesses abundant permanent energy sources. Many substances, such as water, stones, and various CBEs, spontaneously or actively absorb energy from these sources in accordance with some well-known principles of physics and chemistry, such as laws of Newtonian mechanics, laws of thermodynamics (e.g., the second law of thermodynamics, namely that heat can spontaneously flow from hot objects to cold objects, but cannot flow reversely), and laws of organic chemistry [
27,
28]. The energy absorption of CBEs can give rise to a spectrum of transformations, including temperature increases and organic synthesis. These organic synthesis processes transform certain H1-CBEs, such as HCN, CO2, and CH3, along with other substances, into H2-CBEs, like amino acids, nucleotides, and monosaccharides [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15], and certain H2-CBEs, along with other materials, into H3-CBEs, like proteins, nucleic acids, and lipids [
7,
8,
9]. Some H3-CBEs and other molecules form H4-CBEs (unicellular organisms such as bacteria) through some energy-absorbing organic synthesis reactions and energy-consuming physical movements. Some H4-CBEs and other materials form H5-CBEs (multi-cellular organisms, such as birds and trees) through some energy-absorbing organic synthesis reactions and energy-consuming physical movements. For example, some fertilized eggs and related substances develop into adult elephants through many energy-absorbing organic synthesis reactions and many energy-consuming physical movements. Some animal individuals form animal social collectives (H6-CBEs) with the support of energy primarily derived from their food metabolism through organic decomposition reactions. Some human H6-CBEs form multi-hierarchy social collectives (H7-CBEs), such as departments forming colleges and universities, with the support of energy from food and other sources, such as electricity, fossil fuels, and wind energy.
Together, various substances spontaneously or actively absorb energy from the abundant permanent energy sources on Earth, and this constitutes the driving force mechanism of the CBEP, which provides the energy required for the formation of complex CBEs [
29].
5. The Structural Mechanism
It is a fact that some complex CBEs formed through the above energy-absorption processes possess new functions that less complex CBEs do not. For example, bird cells cannot fly, but birds can. This aligns with the core principle of systems theory: the whole is greater than the sum of its parts [
30].
It is also a fact that some structural variations of some complex CBEs can change the functions of the complex CBEs during the cycles of formation and degradation of complex CBEs (the cycles will be explained below in
Section 6). For example, human genomic mutations can lead to some genetic diseases (e.g., thalassemia).
The above two facts stem from the logic that different structures can serve different functions. They constitute the structural mechanisms of the CBEP and provide some new functions of complex CBEs that can facilitate the formation and accumulation of complex CBEs (
Figure 1), as shown by the following examples.
(1) Catalysis of chemical reactions. For instance, after their formation on Earth, various H2-CBEs (e.g., proline [
31]), H3-CBEs (e.g., many proteins), and some CCMs between H2-CBEs and H3-CBEs (e.g., peptides [
32]) can catalyze the synthesis or degradation of certain H2-CBEs and H3-CBEs. These catalyzers facilitate the evolution of CCMs [
33].
(2) Provision of constituent materials. After their accumulation on Earth, various H2-CBEs and H3-CBEs can be used by complex CBEs as raw materials for the construction of more complex CBEs. For instance, H2-CBEs like amino acids or monosaccharides can serve as “bricks” for the construction of H3-CBEs, which can then be utilized as “bricks” for the construction of H4-CBEs.
(3) Capture of constituent materials. For example, plants can capture CO2 from the air and water from their roots to synthesize glucose, and herbivores can browse grasses, which provide various constituent materials for herbivores.
(4) Energy provision. For instance, the breakdown of H2-CBEs and H3-CBEs releases heat or energy, which mammals can utilize for movement or dissipate into their environment.
(5) Active energy absorption. For example, plants' phototropism helps them capture sunlight, and animal metabolism from food provides them with energy from biomass.
(6) Protection of CBEs. For instance, lipids shield H2-CBEs and H3-CBEs that are soluble in lipids from radiation and degradation enzymes, and liposomes safeguard H2-CBEs and H3-CBEs that are water-soluble from certain enzymes.
(7) Direction of the synthesis of some H3-CBEs. For example, nucleic acids (DNA or RNA) can serve as the template to direct the synthesis of DNA, RNA, and proteins, ensuring their precise extension according to specific sequences.
(8) Reproduction For example, H4-CBEs developed the ability to integrate the previous seven functions to replicate themselves.
(9) Sexual selection. For example, female lions mate with male lions who have demonstrated their dominance through fighting.
(10) Non-random mutation. For example, bacteria employ complex genomic structures to reduce random mutations at important genomic sites, and humans employ complex genomic structures to enhance the mutation frequency in immune genes [
34,
35].
(11) Epigenetic changes. Epigenetic changes in gene functions are heritable and can result in alterations to biological traits, despite the fact that the DNA sequence of the genes remains unchanged [
36]. These changes can occur through DNA modifications, histone modifications, chromatin remodeling, and other mechanisms.
(12) Accumulation of knowledge. This function is unique to humans because of their high intelligence, which stems from the intricate structure of their brains. With the accumulation of knowledge, humans created various moral standards and legal systems on which complex human societies were established.
6. The Natural Selection Mechanism
After some relatively simple CBEs form relatively complex CBEs under the action of the energy provided by the driving force mechanism and the functions provided by the structural mechanism, the relatively complex CBEs are usually subject to degradation due to the fifth feature of CBEs listed in
Section 3.3. The degraded complex CBEs, consisting of less complex CBEs and other materials, can be reconstituted into new complex CBEs through the combined action of the driving force and structural mechanisms. Regenerated complex CBEs usually carry some structural changes due to the seventh feature of CBEs listed in
Section 3.3. Therefore, there are cycles of formation and degradation of complex CBEs, and many complex CBEs change over these cycles. These cycles hold the following three mathematical logics.
Logic 1: If the total formation of a complex CBE exceeds its total degradation, this complex CBE persists on Earth, aligning with the phrase “survival of the fit”.
Logic 2: If the ratio of the formation to degradation of a complex CBE exceeds 1 (or falls below 1) over a period, the quantity of this CBE is increasing (or decreasing) during that period.
Logic 3: If the ratio of the formation to degradation of complex CBE A is greater than that of complex CBE B over a period, the ratio of CBE A versus CBE B in quantity is increasing during that period.
The above three mathematical logics constitute the natural selection mechanism, which has the following features:
(1) Natural selection in the CBET stems from mathematical logics, while natural selection in previous theories stems from survival competition or reproduction competition. The mathematical logics constitute the essence of natural selection, while survival competition or reproduction competition is the phenomenon of natural selection in the biosphere.
(2) Natural selection in the CBET applies to the whole CBEP, while natural selection in previous theories is largely restricted to organisms or biological evolution.
(3) The overall performance of the formation and maintenance (OPFM) of a complex CBE determines its fitness in natural selection according to the CBET, and the OPFM itself is determined not by a single component, a single trait, or a single hierarchy of the complex CBE, but by all components, all traits, and all hierarchies of the complex CBE [
37]. This suggests that all components, all traits, and all hierarchies of complex CBEs are under natural selection. In contrast, natural selection in Darwin's theory and the Modern Synthesis frequently highlights the effect of a single mutation or trait [
17]. The notion that the OPFM of a complex CBE determines the results of natural selection or the fitness of CBEs indicates that human individuals or groups should highlight their all-round development. Meanwhile, certain traits of complex CBEs, such as the running ability of antelopes, the group collaboration of wolves, and human individual expertise, can significantly enhance their OPFM. Therefore, natural selection also highlights specialized development.
(4) Natural selection in the CBET exhibits inclusiveness because, according to Logic 1 stated above, a complex CBE can persist on Earth if its rate of formation exceeds its rate of degradation, regardless of whether its OPFM is lower than that of its parents or other CBEs, whether it has some disadvantages, and whether it has encountered any changes. Natural selection allows organisms to have some disadvantages if their OPFM is sufficient. For example, many people carry gene mutations associated with genetic diseases. This further suggests that some biological traits (e.g., the long necks of giraffes) may be advantageous, neutral, or disadvantageous in natural selection. Additionally, the inclusiveness of natural selection is in line with the abundance of biological diversity and the abundance of neutral mutations in genomes, as these mutations have minimal impact on the OPFM of organisms [
17]. In contrast, previous theories emphasized the fierce competition inherent in natural selection, which was expressed as “survival of the fittest” in Darwin's theory and “gradual replacement of populations with those carrying advantageous mutations” in the Modern Synthesis [
17].
(5) Natural selection in the CBET can provide an interpretation for some macroevolution events. For example, the inclusiveness of natural selection in the CBET suggests that sympatric speciation could occur because different mutants with distinct trait combinations can all maintain sufficient OPFM within the same ecological niche and area, particularly when their populations are far from saturation in the area, like in scenarios of adaptive radiation [
38]. For example, antelopes and buffaloes have different traits to confront carnivores, which all provide them with sufficient OPFM in natural selection, and hence they could complete their speciation in the same ecological niche. Previously, it was assumed that sympatric speciation within the same ecological niche was impossible [
39]. Moreover, organisms, including multicellular organisms and endothermic animals, are not inherently fitter than inanimate materials, unicellular organisms, or ectothermic animals, yet they can all maintain sufficient OPFM (i.e., their formation exceeds their degradation), which allows them to persist and evolve.
(6) Natural selection in the CBET is influenced by both inheritable and non-inheritable factors. For example, genetic factors can lead to some individuals having exceptional traits that enhance their OPFM, while non-heritable factors such as education and vaccination can also increase an individual's OPFM. This underscores the importance of considering both innate advantages and acquired strengths. Additionally, epigenetic changes, whether heritable or not, can affect an organism's OPFM and, consequently, shape the dynamics of natural selection [
40].
(7) Natural selection in the CBET is influenced by the environment. Whether an HHCBE has sufficient OPFM depends not only on its own characteristics but also on the environment. Therefore, organisms must adapt to their environment or migrate to more suitable areas for survival and reproduction. Similarly, humans should work to protect the environment.
(8) Natural selection in the CBET involves competition and progressiveness, as suggested by Logic 2 and Logic 3 stated above. Competition results in the accumulation of beneficial changes (positive selection) and the elimination of detrimental changes (negative selection) in relation to the OPFM of complex CBEs. The combined impact of positive and negative selection is a continuous process of optimizing the inner structures, enhancing orderliness, and fostering collaboration within the relevant complex CBEs, which exhibits a progressive tendency of the CBEP. This aligns with the principles of Darwin's theory and the Modern Synthesis, emphasizing that the collaboration of various components, traits, and hierarchies inside organisms is crucial for successful natural selection in the context of biological evolution.
7. The CBEP from the Lens of the CBET
7.1. The Core Viewpoints of the CBET
As elucidated in
Section 3,
Section 4,
Section 5 and
Section 6, due to some principles of physics and chemistry and special features of Earth and CBEs, there are three mechanisms in nature that underpin the CBEP: the driving force mechanism provides energy for the CBEP, the structural mechanism generates new functions (e.g., catalysis and reproduction) for the CBEP, and the natural selection mechanism accumulates orderliness for the CBEP. The synergistic effects of these three mechanisms drive the progression from chemical to biological and social evolution, characterized by the escalating hierarchy of CBEs and the increase in the quantity, diversity, and orderliness of HHCBEs. These are the core viewpoints of the CBET (
Figure 1,
Table 2).
The interplay of these three mechanisms indicates that the CBEP has a significant impact on its own development. In other words, the CBET exhibits a positive feedback loop that intensifies over time. The CBEP acquires and prepares materials for construction and protection, stores energy, and develops catalysts and novel functions to support the subsequent stages of the CBEP. Additionally, the CBEP significantly alters Earth's surface and its own environment, creating opportunities, fostering competition, and potentially leading to disasters [
41]. For example, plants change the color of Earth and provide opportunities for animals and fungi. The increase in photosynthetic bacteria led to the Great Oxidation Event around 2500 million years ago, likely resulting in a significant increase in the concentration of oxygen in the air and the extinction of anaerobic bacteria [
17,
42].
The CBET describes the mechanisms of the CBEP from a panoramic view. Although the CBET cannot provide details for each event of the CBEP during the past billions of years, which should be investigated using some approaches from physics, chemistry, biology, geology, or the social sciences, it can provide the general direction for these investigations.
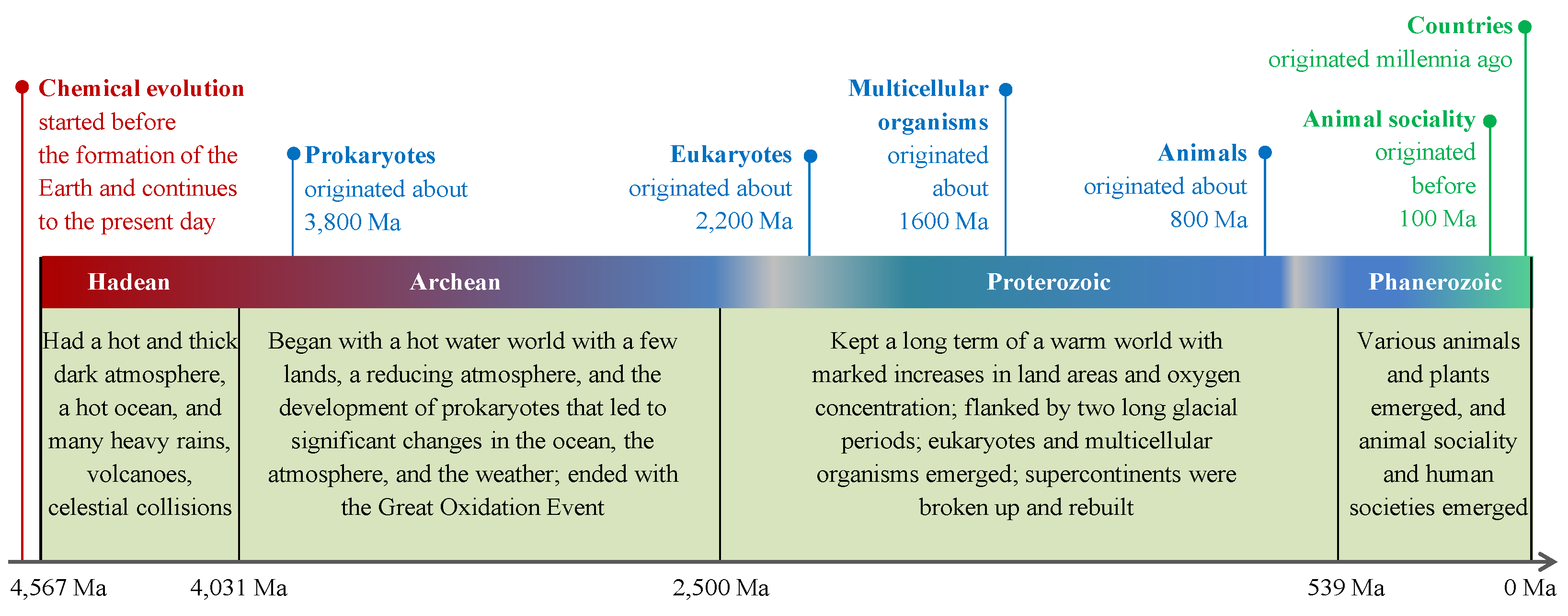
Earth formed around 4.6 billion years ago. Its history has been divided into four eons: Hadean, Archean, Proterozoic, and Phanerozoic [
43] (
Figure 2), and it has experienced chemical evolution, biological evolution, and social evolution, as elucidated below.
7.2. Chemical Evolution from the Lens of the CBET
Chemical evolution is the first phase of the CBEP and represents the origin and development from small CCMs to large CCMs (H3-CBEs). Chemical evolution began before the formation of Earth and continues to the present day. According to current cosmological understanding, H0-CBEs (carbon atoms) on Earth were formed within the interiors of giant or supergiant stars. These atoms were scattered into space as dust during the explosive deaths of these stars in the form of powerful and luminous supernovae. The dust from these supernovae events eventually coalesced to form the Sun, Earth, and other celestial bodies within our solar system [
24].
During and after the formation of Earth, H0-CBEs combined to form H1-CBEs (e.g., carbon dioxide, methane, and hydrogen cyanide). Through heat-absorbing chemical reactions, these H1-CBEs gave rise to a multitude of distinct H2-CBEs, a process that is widely accepted in modern science [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. The prebiotic chemical synthesis routes for various H2-CBEs found in organisms, such as amino acids, nucleotides, and monosaccharides, have been experimentally validated under geologically plausible and biologically relevant conditions in laboratories [
10,
11,
12,
13,
14,
15]. Moreover, myriad distinct H2-CBEs have been identified in meteorites, with mass spectrometry analysis of the Murchison meteorite, which fell in Australia in 1969, suggesting the presence of possibly millions of distinct CCMs [
44]. This evidence supports the hypothesis that myriad distinct H2-CBEs formed through heat-absorbing synthesis reactions prior to Earth's formation.
The prebiotic chemical synthesis routes for H3-CBEs, such as proteins, nucleic acids, lipids, and polysaccharides, which are essential in organisms, have been the subject of exploration for decades, resulting in significant advances. For instance, studies have shown that elements such as phosphorus, boron, and others can aid in the polymerization of proteins and nucleic acids in a prebiotic setting [
6,
7,
8].
The CBET posits that, as a result of the structural mechanism, there should have been an evolution of catalysts from small organic or inorganic molecules to intermediate CCMs, and then to large CCMs, for the polymerization of H3-CBEs with the efficiency and specificity of the catalysts increasing as the structural complexity of the catalysts increased. Consequently, a multitude of distinct proteins, nucleic acids, and other CBEs could have accumulated before the advent of life.
Natural selection can enhance the efficiency of the chemical synthesis of H2-CBEs and H3-CBEs in the inanimate world and the biosphere. However, the total amount of H2-CBEs and H3-CBEs on Earth is influenced by multiple factors. For example, asteroid impacts, volcanic eruptions, glacial periods, and ecosystem destruction can all lead to mass extinctions of organisms and mass reductions in the populations of H2-CBEs and H3-CBEs [
17,
45].
7.3. Biological Evolution from the Lens of the CBET
Biological evolution is the second phase of the CBEP and represents the origin and development of life (H4-CBEs and H5-CBEs). The myriad distinct H2-CBEs and H3-CBEs that emerged on Earth before the origin of life could spontaneously form myriad multiple-molecule structures due to the actions of wind, water flow, evaporation, and other factors. Among these structures, some had one of the first seven functions listed in
Section 5, and a very few had all these seven functions, or the self-reproduction function, due to the complex collaboration of various molecules (Section 5.2). These rare CBEs constituted the first batch of H4-CBEs, which possibly originated at seabeds near hydrothermal vents in the ocean [
46]. One of them passed natural selection and became the Last Universal Common Ancestor (LUCA) of all living things. LUCA could have hundreds of genes [
47]. The possibility of the abiogenesis of H4-CBEs, including LUCA, has been supported by successful experiments regarding the synthesis of viruses and H4-CBEs [
48,
49].
Billions of years after the origin of LUCA, myriad variants of H4-CBEs accumulated on Earth, and some of them became eukaryotes in the early Proterozoic eon [
50]. In the middle Proterozoic eon myriad, variants of eukaryotes accumulated on Earth, and some of them became multicellular organisms (H5-CBEs) [
51]. As elucidated in
Section 6, the origin of H4-CBEs, H5-CBEs, and various species (e.g., amphibians [
34]) was not because they were fitter than their previous taxa, but because they were fit in natural selection: they can obtain sufficient materials and heat or energy to reproduce and maintain themselves due to their complex structures, although they are vulnerable compared to many inanimate materials.
Due to the variability of complex CBEs and the inclusiveness of natural selection, biological evolution has led to the continuous growth of species.
Among the thousands of catalytic molecules in an organism, only a few molecules, such as ribozymes, thrombin, and hammerhead ribozymes, are autocatalyzers that catalyze a step in their own production process. Moreover, no large CCMs have been found that can catalyze all steps of their own synthesis. For example, ribozymes catalyze the formation of ribozymes, but they only catalyze the incorrect folding of ribozymes and cannot catalyze the many steps in the synthesis of ribozymes from certain amino acids in a specific sequence. In biology, the reproductive function of H4-CBEs comes from the collaboration of various molecules and many catalytic molecules that do not catalyze their own synthesis (termed allocatalyzers in this article). Therefore, H4-CBEs are not the hyper-cycle systems composed of autocatalytic molecules hypothesized by Manfred Eigen in the 1970s [
16], but the hyper-cycle systems formed by the collaboration of various allocatalytic molecules with many other molecules, including those providing energy, guiding the precise synthesis of specific molecules, and protecting these molecules. The complex cooperative relationships between these molecules achieve the first seven functions listed above in
Section 5, and these functions collectively achieve the function of reproducing offspring of H4-CBEs. The organic large molecules hypothesized by Manfred Eigen to exist in the hyper-cycle system with both autocatalytic and allocatalytic effects, capable of undergoing mutations, have not yet been discovered in the world. For the same reasons, the RNA world hypothesis, which overestimated the incomplete autocatalytic property of RNA and overlooked the collaboration of various molecules in the origin of life [
52], is questionable.
7.4. Social Evolution from the Lens of the CBET
Social evolution is the third phase of the CBEP and represents the origin and development of animal or human social collectives (H6-CBEs and H7-CBEs), which have the features of the collaboration of animal or human individuals with different duties working for the collectives.
Fossils and molecular clocks both suggested that animals possibly emerged on Earth 800 million years ago [
53]. Animals actively search for and consume food, which provides them with constituent materials and heat or energy. Possibly 100 million years ago, some insects established their social collectives [
54,
55]. The increased complexity in gene regulation and chemical communication is important to the origin of sociality in insects [
54,
55,
56], which coincides with the structural mechanism of the CBET.
Sociality has been established in around 24,000 species of insects and some species of crustaceans and mammals, which constitute separate events in social evolution [
54,
55]. Sociality is widespread in Hymenoptera (ants, bees, and wasps) and Blattodea (termites). A typical social collective has a queen and a few reproductive males, who take on the roles of the sole reproducers, and other individuals act as soldiers and workers who work collaboratively to create a living situation favorable for the brood. Such closely collaborative collectives can be viewed as superorganisms, while multicellular organisms can be viewed as social collectives of cells [
55], and unicellular organisms as social collectives of molecules.
Animal social collectives have complex functions stemming from their complex structures. They significantly reduce intra-population competition and struggles, as well as utilize collective advantages to obtain relevant materials and energy to reproduce and maintain them and confront natural selection. Consequently, although sometimes social animals require individuals to sacrifice their freedom and even their lives for the benefit of others, social animals have strong natural selection advantages due to the collaboration within animal social collectives, and they typically have significantly longer lifespans compared to their counterparts without sociality within the same taxa [
54,
55,
56,
57]. For example, the naked mole rat (Heterocephalus glaber) living in society has a lifespan of up to 30 years, several times longer than that of other rodents. Moreover, they reproduce themselves through the reproduction of a few strong individuals and cooperative brood care, and this specialized breeding can easily accumulate beneficial changes and eliminate harmful ones. On the other hand, the natural selection advantages of animal social collectives also lead to sometimes intense competition or conflicts between animal social collectives. For instance, battles between ant colonies often result in the slaughter of numerous ants [
58].
H6-CBEs have a single management hierarchy. For example, no ant individual controls multiple colonies of ants in the same location.
Due to the complex brains, bipedal bodies, unique vocal cords, and other special structures of humans, humans possess high intelligence, language and written communication, knowledge accumulation, and collaboration capabilities. Building upon these advantages, humans have established universities, armies, banks, countries, and various other social collectives (H7-CBEs) that have multiple hierarchies in management.
In H7-CBRs, low-hierarchy social collectives adhere to the laws of physics, chemistry, and biology, as well as the rules of social collectives, serving high-hierarchy social collectives. High-hierarchy social collectives benefit from the collaboration between low-hierarchy social collectives, enabling them to acquire resources, defend themselves, reproduce, and decrease internal competition and conflicts more efficiently. Therefore, although sometimes human collectives may need to sacrifice individual freedom or even the lives of certain individuals (such as soldiers, policemen, or firefighters) to protect social collectives and other individuals, humans generally have longer lifespans compared to other primates.
The natural selection advantages of human social collectives usually increase as the hierarchy of the collective rises. This is why humans worldwide have established multi-hierarchy social collectives largely along the line from clans to tribes, tribal alliances, nations, and national alliances. However, the natural selection advantages of human social collectives can also intensify competition and conflicts between higher-hierarchy human societies, including wars. In 2022, global military spending reached
$2.2 trillion, and 238,000 people died as a result of wars [
59]. Advances in technology, such as the development of nuclear weapons and artificial intelligence, have significantly increased the destructive potential of international conflicts, posing a threat to humanity and Earth. These enormous military expenditures and casualties as well as the threats to humanity and Earth could be circumvented if a global and harmonious human social collective were established, like the fact that in a harmonious country, there are no economic losses or human deaths due to internal wars. Therefore, the CBET asserts that one of the rational ultimate objectives of the CBEP is to unite all countries into a single harmonious social collective, which, in essence, requires that the rational interests of most human individuals and human social collectives be respected in social rules and management. Accordingly, the CBET advocates for the peaceful and harmonious development of human society.
7.5. The Natural Roots of Multiple Important Social Notions
From the view of the CBET, the natural roots of the social notions of all-round development, inclusiveness, collaboration, competition, inherited advantages, acquired strengths, and harmonious and peaceful development of human society have been clarified in
Section 6 and Section 7.4. The natural roots of selfishness, altruism, restriction of freedom, and freedom are elucidated below.
In the survival competition of natural selection, selfishness is essential for myriad animals to obtain adequate constituent materials, energy, suitable environments, and mating opportunities to maintain their lives and reproduce themselves. Meanwhile, altruism is also widespread throughout the CBEP: many molecules are the catalyzers, energy-providers, or constituent materials for the synthesis of other molecules, including H2-CBEs and H3-CBEs; many molecules in H4-CBEs (e.g., bacteria) help the passage of nucleic acids to the next generation; many cells in H5-CBEs (e.g., humans) help the passage of reproductive cells to the next generation; many animal individuals in H6-CBEs (e.g., ant colonies) help the reproduction of a few reproductive individuals in the collectives; and many human individuals in H7-CBEs (e.g., soldiers, policemen, and firefighters in a country) sacrifice themselves for the benefit of the whole collectives.
Restriction of the freedom of LHCBEs in HHCBEs is also widespread because HHCBEs are established on the collaboration of their inner LHCBEs, which requires the LHCBEs to sacrifice their freedom for the collaboration. However, the freedom of LHCBEs increases with the increase in the hierarchies of CBEs. For example, many atoms can hardly conduct relative motion inside H1-CBEs and H2-CBEs, but they can conduct nanometer-sized relative motion in H3-CBEs. Many molecules can conduct micrometer-sized relative motion inside cells. Many cells can conduct meter-sized movement in multicellular organisms, and many animal individuals in animal groups can move relatively freely in certain areas. As for many animals, freedom is important for them to search for and consume sufficient food, fight against predators, obtain mating opportunities, and reproduce themselves. Furthermore, freedom is important for animals to move to more suitable environments.
8. Reliability of the CBET
(1) The CBET, which harbors no weird viewpoints, does not rely on obscure concepts or complex mathematical formulas (
Figure 1 and
Table 2), and consequently, its logical reasoning is easily evaluated.
(2) The factors highlighted in the CBET, such as the special features of Earth, the special features of carbon atoms and other CBEs, energy, chemical reactions, the new functions stemming from the increase in structural complexity, and natural selection, are all critical for the CBEP. In contrast, previous theories only addressed a portion of these factors.
(3) The above critical factors are integrated logically in CBET with widely accepted knowledge in physics, chemistry, biology, geology, astronomy, and social sciences, rather than with any novel laws in physics, novel observations, or novel experiments.
(4) The development of humans from inanimate materials (H1-CBEs to H3-CBEs) to fertilized eggs (H4-CBEs), individuals (H5-CBEs), and teachers in some departments (H6-CBEs) of certain universities (H7-CBEs) can be considered a miniature of the CBEP, which can test the validity of the core views of the CBET: the driving force mechanism provides energy for the process; the structural mechanism provides functions for the process; and the natural selection mechanism has accumulated various beneficial genetic mutations and acquired strengths (e.g., education and vaccination) for the human individuals, and various principles of physics and chemistry, features of Earth, and features of CBEs are important in origin of the three mechanisms.
(5) As shown below in Section 9 and
Table 3, the CBET provides better explanations for the origin of life and some other macroevolution issues and accommodates multiple evolutionary facts that cannot be integrated into any previous theories. This also supports the reliability of the CBET.
9. Novelties of the CBET
In science, the CBET elucidates that the CBEP arises from the synergistic action of three mechanisms, which provide the CBEP with energy, functions, and orderliness, respectively. The CBET thus answers the core science question of why inanimate matter evolved into complex organisms and societies in a novel, direct, explicit, and relatively comprehensive way. In contrast, the three phases of the CBEP, namely chemical evolution, biological evolution, and social evolution, were usually investigated separately in previous theories, and few theories have explicitly interpreted the mechanisms of the CBEP from a panoramic view, although some significant efforts on this topic have been published [
5,
60]. Moreover, only one mechanism, such as natural selection in Darwin's theory [
17], entropy dissipation into the surroundings in Schrödinger's negative entropy notion [
3], self-organization in Prigogine's dissipative structure theory [
4], the constructal law [
60], the maximum entropy production principle [
61], or the free-energy principle [
62], was proposed to explain the CBEP in previous theories or hypotheses. These one-mechanism theories cannot explicitly or comprehensively explain the CBEP. Moreover, they overlooked or even rejected the crucial role of natural selection, except for Darwin's theory and the Modern Synthesis. Although Darwin's theory and the Modern Synthesis highlighted the importance of natural selection, they did not clarify why organisms can exist. They assumed that natural selection, gene drift, competition, or mutations are the driving forces of evolution [
63,
64,
65,
66,
67], but these factors require energy and do not provide energy for the CBEP.
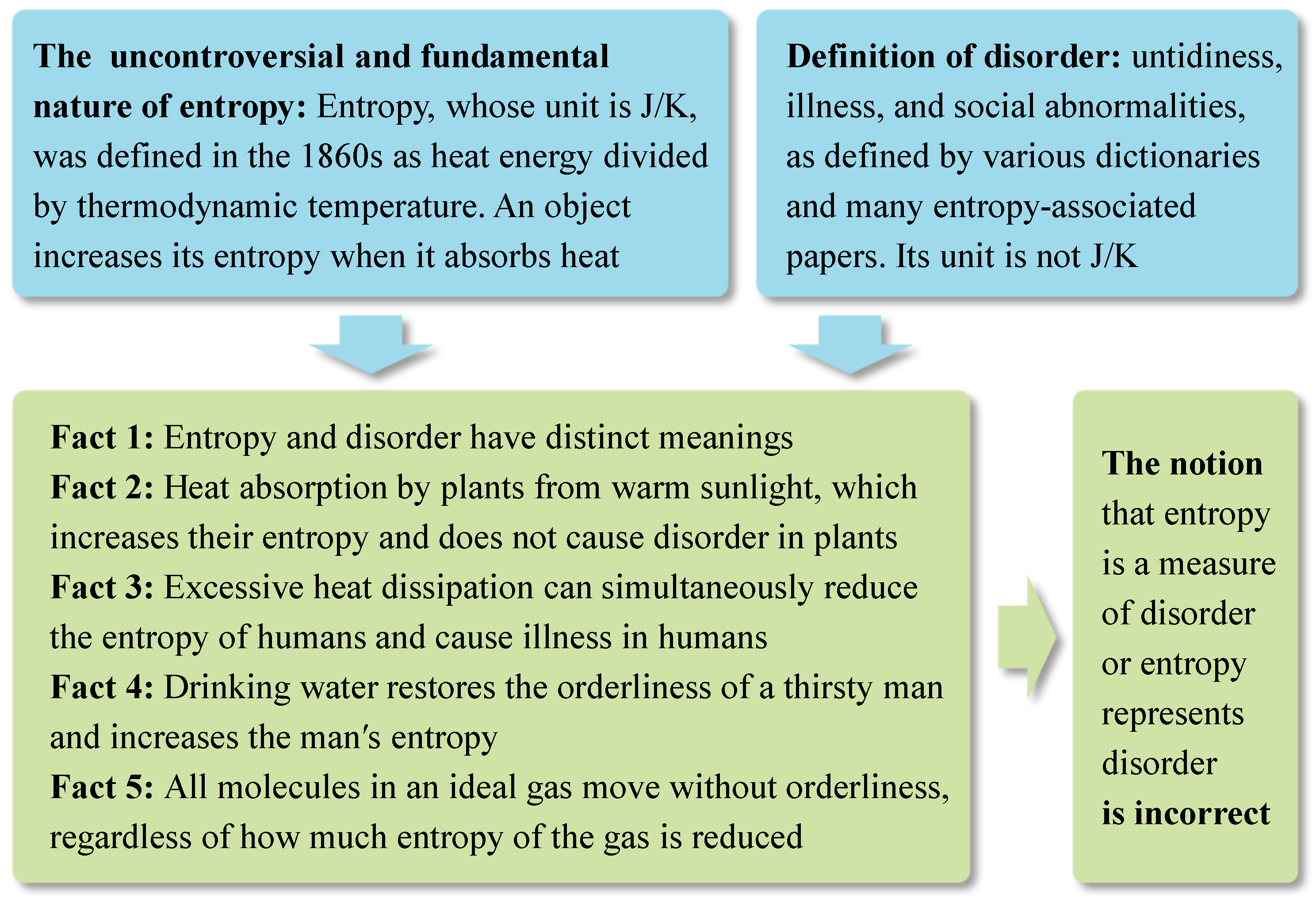
In physics, the CBET could challenge some widely accepted viewpoints regarding thermodynamics and evolution. The second law of thermodynamics, which states that heat can spontaneously flow from hotter to colder objects and not the reverse, can also be mathematically expressed as the entropy of isolated systems increasing over time. Since entropy is commonly assumed to be a measure of disorder and the entire universe can be considered as an isolated system, the law indicates that the universe tends to become more and more disordered, which contradicts evolution, a natural process characterized by an increase in orderliness [
3,
4,
61,
62]. Creationists (including some scientists) have exploited this perceived contradiction to argue for the existence of divine entities [
2]. Some influential theories, such as Schrödinger's negative entropy theory and Prigogine's dissipative structure theory, have attempted to reconcile this contradiction by suggesting that open systems (like organisms) can gain orderliness through the dissipation of entropy into their environment. [
3,
4]. These theories accepted the notion that entropy represents disorder and overlooked the important roles of energy, chemical reactions, the special features of carbon atoms and other CBEs, and natural selection. In contrast, the CBET highlights energy, chemical reactions, the special features of carbon atoms and other CBEs, and natural selection. It embraces the new notion that entropy and disorder have distinct meanings, and hence entropy cannot represent disorder [
5,
68] (
Figure 3). Therefore, the second law of thermodynamics does not contradict evolution. Moreover, the CBET clarifies that the second law of thermodynamics is highly associated with the driving force of evolution (
Section 4,
Section 5 and
Section 6), which refutes a key argument used by creationism and coincides with some research [
5,
60,
68].
In biology, as elucidated in
Section 6, the CBET reveals the driving force of biological evolution and the mathematical essence of natural selection and provides more comprehensive explanations regarding the targets of natural selection, biological altruism, biological diversity, and some macroevolution issues, such as the origin of life, multicellular organisms, endothermic animals, and sympatric speciation. Moreover, natural selection, non-random mutations, neutral mutations, epigenetic changes, and acquired strengths, which cannot be integrated into any previous evolutionary theory, are integrated into the cohesive framework of the CBET, as elucidated in
Section 5 and
Section 6.
In the social sciences, the CBET reveals the natural roots of multiple pivotal and seemingly paradoxical social notions, such as inclusiveness and competition, altruism and selfishness, freedom and restriction, inherited advantages and acquired strengths, as well as specialized development and all-around development. Therefore, the CBET advocates for the balanced development of human society. The CBET also elucidates the imperative of the integration of all countries into a single harmonious social collective. In contrast, previous evolutionary theories highlight selfishness, competition, and the elimination of those less advantageous in certain traits [
1]. These biased notions have historically been employed to rationalize colonialism, slavery, racism, and genocide [
69].
10. Conclusions and Perspectives
This article establishes the CBET, which provides novel, direct, explicit, and relatively comprehensive explanations for the evolution of inanimate matter on Earth into complex organisms and social structures. The theory elucidates three mechanisms shared by chemical evolution, biological evolution, and social evolution. It demonstrates the absence of conflict between the second law of thermodynamics and evolution, thereby refuting a key argument used by creationism and unifying biology with physics and chemistry. It challenges Schrödinger's negative entropy notion, Prigogine's dissipative structure theory, Eigen's hypercycle theory, and the RNA world hypothesis. It reveals the mathematical essence of natural selection and provides more comprehensive explanations for natural selection and some other evolutionary phenomena. The theory uncovers the underlying natural roots of multiple crucial social notions, such as inclusiveness, altruism, collaboration, and freedom, besides selfishness, competition, and restriction, thereby bridging the natural sciences with the social sciences.
The CBET can guide natural scientific research. For instance, on chemical evolution and the origin of life, this theory suggests searching for some CCMs (e.g., peptides) that can effectively catalyze the synthesis of proteins, nucleic acids, or other organic macromolecules associated with life. It also suggests investigating collaborative relationships among various molecules in the context of life's origin. It recommends a panoramic perspective for research on natural selection, macroevolution, and social behaviors in animals.
The CBET can guide social scientific research and social development because it uncovers the underlying natural roots of multiple crucial social notions and advocates for the balanced, harmonious, and peaceful development of human society, as well as the integration of all countries into a single harmonious social collective.
Acknowledgments
The authors thank Meng Yang for her various constructive comments. The authors thank Yiqing Chen for her contribution to Sections 1, 6, and 7 as well as the figures. This work was supported by the High-Level Talent Fund of Foshan University (No. 20210036).
Conflict of interest
The authors declare no competing interests.
References
- Xie, P. The aufhebung and breakthrough of the theories on the origin and evolution of life; Science Press, 2014. [Google Scholar]
- Schreiber, A.; Gimbel, S. Evolution and the second law of thermodynamics: Effectively communicating to non-technicians. Evo Edu Outreach 2010, 3, 99–106. [Google Scholar] [CrossRef]
- Schrodinger, E. What is life; Cambridge University Press, 2012. [Google Scholar]
- Prigogine, I. Time, structure and fluctuation (Nobel Lecture). Science 1978, 201, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Chen, J.W. Root of science—the driving force and mechanisms of the extensive evolution; Science Press, 2000. [Google Scholar]
- Oparin, A.I. Chemistry and the origin of life. R. Inst. Chem. Rev. 1969, 2, 1–12. [Google Scholar] [CrossRef]
- Guo, X.; Fu, S.; Ying, J.; Zhao, Y. Prebiotic chemistry: a review of nucleoside phosphorylation and polymerization. Open Biol 2023, 13, 220234. [Google Scholar] [CrossRef] [PubMed]
- Sumie, Y.; Sato, K.; Kakegawa, T.; Furukawa, Y. Boron-assisted abiotic polypeptide synthesis. Commun. Chem. 2023, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.; De Decker, Y.; Sojo, V.; Hudson, R. Quantifying catalysis at the origin of life. Chemistry 2023, 29, e202301447. [Google Scholar] [CrossRef] [PubMed]
- Nogal, N.; Sanz-Sánchez, M.; Vela-Gallego, S.; Ruiz-Mirazo, K.; de la Escosura, A. The protometabolic nature of prebiotic chemistry. Chem Soc Rev 2023, 52, 7359–7388. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, C.; Shvetsova, A.; Skorda, F.; Lopez, A.; Fiore, M. The origin and early evolution of life: Homochirality emergence in prebiotic environments. Astrobiology 2023, 23, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M. Prebiotic chemistry and life's origin; Royal Society of Chemistry, 2022. [Google Scholar] [CrossRef]
- Anna Neubeck, A.; McMahon, S. Prebiotic chemistry and the origin of life; Springer, 2021. [Google Scholar] [CrossRef]
- Farías-Rico, J.A.; Mourra-Díaz, C.M. A short tale of the origin of proteins and ribosome evolution. Microorganisms 2022, 10, 2115. [Google Scholar] [CrossRef]
- Ershov, B. Natural radioactivity and chemical evolution on the early Earth: Prebiotic chemistry and oxygenation. Molecules 2022, 27, 8584. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. Stages of emerging life—Five principles of early organization. J. Mol. Evo. 1982, 19, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Futuyma, D.J.; Kirkpatrick, M. Evolution; Sinauer Associates, 2017. [Google Scholar]
- White, L.A. The evolution of culture: the development of civilization to the fall of Rome; Routledge, 2010. [Google Scholar] [CrossRef]
- Richerson, P.J.; Christiansen, M.H. Cultural evolution: Society, technology, language, and religion; The MIT Press, 2013. [Google Scholar]
- Laland, K.N. Darwin's unfinished symphony: How culture made the human mind; Princeton University Press, 2017. [Google Scholar]
- Seager, S. Exoplanet habitability. Science 2013, 340, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Charette, M.A.; Smith, W.H.F. The volume of Earth's ocean. Oceanography 2010, 23, 112–114. [Google Scholar] [CrossRef]
- Carbon. Available online: https://en.wikipedia.org/wiki/carbon (accessed on 09 April 2024).
- Roston, E. The carbon age: How life's core element has become civilization's greatest threat; Walker & Company, 2008. [Google Scholar]
- Li, Z.H.; Bailleul, A.M.; Stidham, T.A.; Wang, M.; Teng, T. Exceptional preservation of an extinct ostrich from the Late Miocene Linxia Basin of China. Vertebrata PalAsiatica 2021, 59, 229. [Google Scholar] [CrossRef]
- Heck, P.R.; Greer, J.; Kööp, L.; et al. Lifetimes of interstellar dust from cosmic ray exposure ages of presolar silicon carbide. Proc. Natl. Acad. Sci. USA 2020, 117, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, C.; Sonntag, R.E. Fundamentals of thermodynamics; Wiley, 2022. [Google Scholar]
- DeVoe, H. Thermodynamics and chemistry. Available online: https://www2.chem.umd.edu/thermobook/v10-screen.pdf (accessed on 09 April 2024).
- Martin, W.F.; Sousa, F.L.; Lane, N. Energy at life's origin. Science 2014, 344, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- von Bertalanffy, L. General system theory: Foundations, development, applications; George Braziller, 1968. [Google Scholar]
- Morrell, D.G. Catalysis of organic reactions; CRC Press, 2019. [Google Scholar]
- Stone, E.A.; Cutrona, K.J.; Miller, S.J. Asymmetric catalysis upon helically chiral loratadine analogues unveils enantiomer-dependent antihistamine activity. J. Am. Chem. Soc. 2020, 142, 12690–12698. [Google Scholar] [CrossRef]
- de Graaf, R.; De Decker, Y.; Sojo, V.; Hudson, R. Quantifying catalysis at the origin of life. Chemistry 2023, 29, e202301447. [Google Scholar] [CrossRef]
- Fitzgerald, D.M.; Rosenberg, S.M. What is mutation? A chapter in the series: How microbes ”jeopardize” the modern synthesis. PLoS Genet. 2019, 15, e1007995. [Google Scholar] [CrossRef]
- Olivieri, D.N.; Mirete-Bachiller, S.; Gambón-Deza, F. Insights into the evolution of IG genes in amphibians and reptiles. Dev. Comp. Immunol. 2021, 114, 103868. [Google Scholar] [CrossRef]
- Sabarís, G.; Fitz-James, M.H.; Cavalli, G. Epigenetic inheritance in adaptive evolution. Ann. N. Y. Acad. Sci. 2023, 1524, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, Y. Variation in the analysis of positively selected sites using nonsynonymous/synonymous rate ratios: An example using influenza virus. PLoS One 2011, 6, e19996. [Google Scholar] [CrossRef]
- McGee, M.D.; Borstein, S.R.; Meier, J.I.; et al. The ecological and genomic basis of explosive adaptive radiation. Nature 2020, 586, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Ecological factors in speciation. Evolution 1947, 1, 263–288. [Google Scholar] [CrossRef]
- Gómez-Schiavon, M.; Buchler, N.E. Epigenetic switching as a strategy for quick adaptation while attenuating biochemical noise. PLoS Comput Biol 2019, 15, e1007364. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J. The red queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 2009, 323, 728–732. [Google Scholar] [CrossRef]
- Cohen, K.M.; Harper, D.A.T.; Gibbard, P.L.; Car, N. International chronostratigraphic chart. Available online: https://stratigraphy.org/ICSchart/ChronostratChart2023-09.pdf (accessed on 09 April 2024).
- Olejarz, J.; Iwasa, Y.; Knoll, A.H.; et al. The Great Oxygenation Event as a consequence of ecological dynamics modulated by planetary change. Nat. Commun. 2021, 12, 3985. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeonm, R.D.; et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef]
- Percival, L.M.E.; Ruhl, M.; Hesselbo, S.P.; et al. Mercury evidence for pulsed volcanism during the end-Triassic mass extinction. Proc. Natl. Acad. Sci. USA 2017, 114, 7929–7934. [Google Scholar] [CrossRef]
- Dodd, M.S.; Papineau, D.; Grenne, T.; et al. Evidence for early life in earth's oldest hydrothermal vent precipitates. Nature 2017, 543, 60–64. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; et al. The physiology and habitat of the last universal common ancestor. Nat. Microb. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Stobart, C.C.; Moore, M.L. RNA virus reverse genetics and vaccine design. Viruses 2014, 6, 2531–2550. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A., 3rd; Chuang, R.Y.; Noskov, V.N.; et al. Design and synthesis of a minimal bacterial genome. Science 2016, 351, aad6253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhu, M.; Knoll, A.; et al. Decimetre-scale multicellular eukaryotes from the 1.56-billion-year-old Gaoyuzhuang formation in North China. Nat. Commun. 2016, 7, 11500. [Google Scholar] [CrossRef] [PubMed]
- Han, T.M.; Runnegar, B. Megascopic eukaryotic algae from the 2.1-billion-year-old negaunee iron-formation, Michigan. Science 1992, 257, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Woltz, C.R.; Tosca, N.J.; Porter, S.M.; Briggs, D.E.G. Fossilisation processes and our reading of animal antiquity. Trends Ecol Evol 2023, 38, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, X.; Shih, C.; Gao, T.; Ren, D. Termite colonies from mid-Cretaceous Myanmar demonstrate their early eusocial lifestyle in damp wood. Natl. Sci. Rev. 2020, 7, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Mera-Rodríguez, D.; Jourdan, H.; Ward, P.S.; Shattuck, S.; Cover, S.P.; Wilson, E.O.; Rabeling, C. Biogeography and evolution of social parasitism in Australian Myrmecia bulldog ants revealed by phylogenomics. Mol. Phyogenet. Evol. 2023, 186, 107825. [Google Scholar] [CrossRef]
- Nowak, M.; Tarnita, C.; Wilson, E. The evolution of eusociality. Nature 2010, 466, 1057–1062. [Google Scholar] [CrossRef]
- Plowers, N. An introduction to eusociality. Nature Education Knowledge 2010, 3, 7. [Google Scholar]
- Moffett, M.W. Adventures among ants; University of California Press, 2010. [Google Scholar]
- SIPRI Military Expenditure Database. Available online: https://www.sipri.org/databases/milex (accessed on 09 April 2024).
- Bejan, A. The principle underlying all evolution, biological, geophysical, social and technological. Philos. Trans. A. Math. Phys. Eng. Sci. 2023, 381, 20220288. [Google Scholar] [CrossRef] [PubMed]
- Ramstead, M.J.D.; Badcock, P.B.; Friston, K.J. Answering Schrödinger's question: A free-energy formulation. Phys. Life Rev. 2018, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Skene, K.R. Systems theory, thermodynamics and life: Integrated thinking across ecology, organization and biological evolution. Biosystems 2024, 236, 105123. [Google Scholar] [CrossRef] [PubMed]
- Rott, P.; Grinstead, S.; Dallot, S.; et al. Genetic diversity, evolution, and diagnosis of sugarcane yellow leaf virus from 19 sugarcane-producing locations worldwide. Plant Dis. 2023, 107, 3437–3447. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, C.; et al. The pig pangenome provides insights into the roles of coding structural variations in genetic diversity and adaptation. Genome Res. 2023, 33, 1833–1847. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, Y.; Yang, S.; et al. Genome-wide identification and analysis of WRKY gene family in Melastoma dodecandrum. Int. J. Mol. Sci. 2023, 24, 14904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Feng, Y. Autotetraploid origin of Chinese cherry revealed by chromosomal karyotype and in situ hybridization of seedling progenies. Plants 2023, 12, 3116. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Liu, D.; et al. Genome-wide analysis of the membrane attack complex and perforin genes and their expression pattern under stress in the Solanaceae. Int. J. Mol. Sci. 2023, 24, 13193. [Google Scholar] [CrossRef]
- Chen, J.M.; Chen, J.W. Disproving two widely accepted notions regarding entropy. Preprints. 2024. https://www.preprints.org/manuscript/202404.0655/v2. [CrossRef]
- Rudman, L.A.; Saud, L.H. Justifying social inequalities: The role of social Darwinism. Pers. Soc. Psychol. B. 2020, 46, 1139–1155. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).