1. Introduction
Why and how did unordered, simple substances on Earth evolve into orderly, complex, and diverse organisms and social organizations? This is a fundamental question in science that has captivated humans for millennia [
1]. Creationists claim that humans, life, Earth, and the whole universe were created by one or more supernatural powers [
2].
Some notions or theories in physics, such as the notion of negative entropy proposed by Nobel laureate Erwin Schrödinger [
3], the dissipative structure theory proposed by Nobel laureate Ilya Prigogine [
4], and the maximum entropy production hypothesis [
5], employed various abstract concepts to interpret the origin of the orderliness of organisms. These notions or theories did not provide explicit answers about evolution.
In chemistry, in the 1920s, Alexander Oparin proposed a hypothesis suggesting that life on Earth originated through a gradual chemical evolution of organic molecules [
6]. Since the 1950s, many experiments have been conducted to investigate how various organic molecules, such as amino acids, monosaccharides, nucleotides, proteins, and nucleic acids, could be synthesized naturally on the prebiotic Earth [
7,
8,
9,
10,
11,
12,
13,
14,
15]. The concept of chemical evolution has been widely accepted, although the mechanism and processes of chemical evolution have not been fully revealed. In the 1970s, the hypercycle theory proposed by Nobel laureate Manfred Eigen assumed that some autocatalytic or self-replicating macromolecules are interconnected in a way that each of them catalyzes the production of its successor, with the last molecule catalyzing the first one [
16]. To date, no examples have been identified to support the hypercycle theory.
In biology, various evolutionary theories have been proposed, including the natural selection hypothesis proposed by Charles Darwin in the 19th century and its updated version, the Modern Synthesis, which emerged in the 20th century [
17]. Darwin's theory elucidated the importance of natural selection, and the Modern Synthesis elucidated the genetic basis underlying it [
17]. These two theories cannot explain some macroevolution issues, such as the origin of life and multicellular organisms, because organisms are no fitter than inanimate materials, and multicellular organisms are no fitter than unicellular organisms [
17].
In the social sciences, some social evolution theories, inspired by Darwin's theory, led to notorious notions, such as social Darwinism, which highlighted fierce competition and justified the policies of colonialism, slavery, racism, and massacre [
18,
19,
20]. These theories have not revealed the natural roots of some key social notions, such as inclusiveness, altruism, and collaboration.
In general, previous evolutionary theories are inexplicit (e.g., some theories in physics and the hypercycle theory) or incomplete (e.g., Darwinism, the Modern Synthesis, and some social evolutionary theories). Furthermore, they did not explicitly unify chemical evolution, biological evolution, and social evolution. Therefore, it is desirable to conduct research to provide an explicit and comprehensive explanation for evolution.
2. Definitions
Driving force refers to the energy that propels changes. For instance, gasoline provides the driving force of fuel-powered cars.
Mechanism is defined as the reason for the development and change of things. The driving force is a mechanism. For example, airplanes have two mechanisms to fly: the airplane has a sophisticated structure that supports flight, and fuel combustion generates energy or driving force to support the airplane to fly.
Carbon-based materials (CBMs) are those substances in which carbon atoms play a major role at the atomic level. There are eight hierarchies of CBMs, as defined below.
H0-CBMs refer to carbon atoms.
H1-CBMs refer to small carbon-containing molecules (CCMs), such as methane and carbon dioxide.
H2-CBMs refer to intermediate CCMs, such as lysine and glucose.
H3-CBMs refer to large CCMs, such as proteins and nucleic acids, composed of H2-CBM residues and some functional groups.
H4-CBMs refer to cells, such as bacteria, composed of various H3-CBMs and other molecules.
H5-CBMs refer to multicellular organisms, such as pines and rabbits, composed of some H4-CBMs (cells) and other materials.
H6-CBMs refer to animal social collectives, such as ant colonies and bee colonies, composed of some animal individuals that closely collaborate for the collective good and have distinct roles within the collectives. H6-CBMs have only one hierarchy in management.
H7-CBMs refer to human social collectives that have multiple hierarchies in management, and low-hierarchy collectives closely collaborate with different duties for high-hierarchy collectives. For example, a university is an H7-CBM as it possesses the hierarchies of departments, colleges, and the whole university in management. Also, an army and a country are H7-CBMs as they have multiple hierarchies in management.
Notably, no clear lines separate these CBM hierarchies. For example, some peptides are between H2-CBMs and H3-CBMs, viruses are between H3-CBMs and H4-CBMs, and lion groups are between H5-CBMs and H6-CBMs.
High-hierarchy CBMs (HHCBMs) and low-hierarchy CBMs (LHCBMs) are defined by comparing their hierarchies. For example, H4-CBMs are HHCBMs compared to H3-CBMs, but they are LHCBMs compared to H5-CBMs. Sometimes LHCBMs refer to H0-CBMs to H3-CBMs, while HHCBMs refer to H4-CBMs to H7-CBMs.
Simple CBMs and complex CBMs are defined by comparing their structural complexity. For example, eukaryotic paramecia are complex CBMs compared to prokaryotic staphylococci, but they are simple CBMs compared with multi-cellular ants. Sometimes simple CBMs refer to H0-CBMs to H3-CBMs, while complex CBMs refer to H4-CBMs to H7-CBMs.
Chemical evolution refers to the evolution from small CCMs to intermediate CCMs and large CCMs, or from H1-CBMs to H2-CBMs and H3-CBMs‘
Biological evolution refers to the origin and evolution of life, namely H4-CBMs and H5-CBMs).
Social evolution refers to the origin and evolution of animal or human social collectives, namely H6-CBMs and H7-CBMs.
The evolution of CBMs refers to the entire evolution process from H1-CBMs to H7-CBMs, covering the three phases of chemical evolution, biological evolution, and social evolution.
The Carbon-Based Evolutionary Theory (CBET) is defined as an evolutionary theory elucidating the mechanisms of the evolution of CBMs.
3. Special features of Earth and CBMs
3.1. Special features of Earth
Feature 1: Earth has abundant energy sources, such as cosmic radiation, sunlight, lightning, geothermal energy, volcanoes, fires, water flow, wind, and the degradation of organic materials [
21].
Feature 2: Earth has abundant water, which weighs around 1.35×10
18 tons [
22].
The energy released from the energy sources on Earth is regulated by the atmosphere, which is over 1,000 kilometers thick, and the abundant water on Earth. This regulation helps to maintain a moderate, widespread, and persistent distribution of energy on Earth and renders Earth an exceptionally rare and hospitable celestial body in astronomy [
21].
Water and energy are essential to the synthesis of organic molecules, the growth of plants, the movement of animals, the reproduction of organisms, and the development of human society. Besides adjusting the energy on Earth, water participates in the formation of all hierarchies of CBMs as an important constituent component and the environment of the formation. Water is also important to maintain the structures and functions of many CBMs. Given the crucial role of water and energy, tropical rain forests boast a greater abundance and variety of organisms compared to tropical deserts or cold regions.
3.2. Special Features of H0-CBMs (Carbon Atoms)
The following specific features of carbon atoms significantly contributed to the evolution of CBMs, which helps to explain why, among all substances on Earth, only CBMs have evolved from simple structures to highly complex structures.
Feature 1: Carbon is abundant on Earth. It has been estimated that the atmosphere, water bodies, and biosphere on Earth contain 9×10
11, 36×10
11, and 5.5×10
11 tons of carbon, respectively [
23,
24]. There is also abundant carbon in stones, coals, petroleum, natural gas, and methane hydrates on Earth.
Feature 2: Carbon atoms can form chemical bonds with a wide range of other atoms and numerous intermediate CCMs that are soluble in water or oil. This is because carbon atoms are smaller than the other atoms (silicon, germanium, tin, and lead) that have four electrons in their valence shells [
23,
24].
Feature 3: Among all elements, carbon is unique in its ability to form long, relatively stable chains of interconnected bonds, which serve as the backbone for numerous large CCMs that are soluble in water. These backbones can bond with atoms such as hydrogen, oxygen, nitrogen, sulfur, phosphorus, halogens, metals, and various groups of atoms known as functional groups. The length, shape, and chirality of the chains all play a role in determining the properties of these large CCMs. No other atoms, including boron and silicon, are capable of forming the backbones of chains in large molecules that are as long, complex, or stable as carbon-based large organic molecules in water [
23,
24]. Because stable, complex, and water-soluble large molecules are essential for the construction of organisms, it is widely accepted that only CBMs have the potential to evolve from simple structures into life [
23,
24].
3.3. Special features of other CBMs (H1-CBMs to H7-CBMs)
Feature 4: Many CBMs are relatively stable on Earth. For example, some proteins have been well preserved in fossils for millions of years [
25], and various H2-CBMs have been preserved longer than proteins in meteorites [
26]. Human individuals can usually live for decades, and some plants can live for millennia.
Feature 5: Many CBMs, along with other materials, can form CBMs one hierarchy higher after energy absorption. For example, CO
2 (belonging to H1-CBMs) and water, after absorbing energy from sunlight, are able to form glucose (belonging to H2-CBMs) in the leaves of plants, and glucose can form polysaccharides (which belong to H3-CBMs) after absorbing heat under the catalysis of amylase. This is further elaborated below in
Section 4 and
Section 7.
Feature 6: Almost all complex CBMs are degradable. Typically, an external factor such as fire, collision, or radiation can degrade a complex CBM by disrupting its component interactions. High temperatures, such as those from mountain fires or asteroid impacts, can accelerate the degradation of complex CBMs [
17]. Usually, it is easier to destroy a complex CBM than to generate it. For example, it takes only minutes with electricity to kill a pig that has been nourished for months. Additionally, HHCBMs cannot be directly formed from CBMs that are two or more hierarchies lower, while HHCBMs can be degraded into CBMs that are two or more hierarchies lower. For example, a living tree (an H5-CBM) can be directly decomposed into carbon atoms (H0-CBMs) and other materials.
Feature 7: Organic synthesis reactions of many H2-CBMs and H3-CBMs are complex, with products varying based on different starting materials and reaction conditions. Even when using the same starting material under identical reaction conditions, the products of these reactions are usually diverse. Furthermore, HHCBMs, ranging from H4-CBMs to H7-CBMs, are multifarious because they result from the recombination of various LHCBMs.
4. The driving force mechanism
As shown in
Section 2, Earth possesses abundant permanent energy sources. Many CBMs and other materials, such as water, and stones, spontaneously or actively absorb energy from these sources in accordance with some principles of classical physics and chemistry that apply to the hierarchies between atoms and the solar system where the evolution of CBMs occurred, such as the laws of Newtonian mechanics, the laws of thermodynamics (e.g., the second law of thermodynamics, namely that heat can spontaneously flow from hot objects to cold objects, but cannot flow reversely), and the laws of inorganic and organic chemistry [
27,
28]. This constitutes the driving force mechanism of the evolution of CBMs, which, as elaborated below, provides the energy required for the formation of complex CBMs [
29].
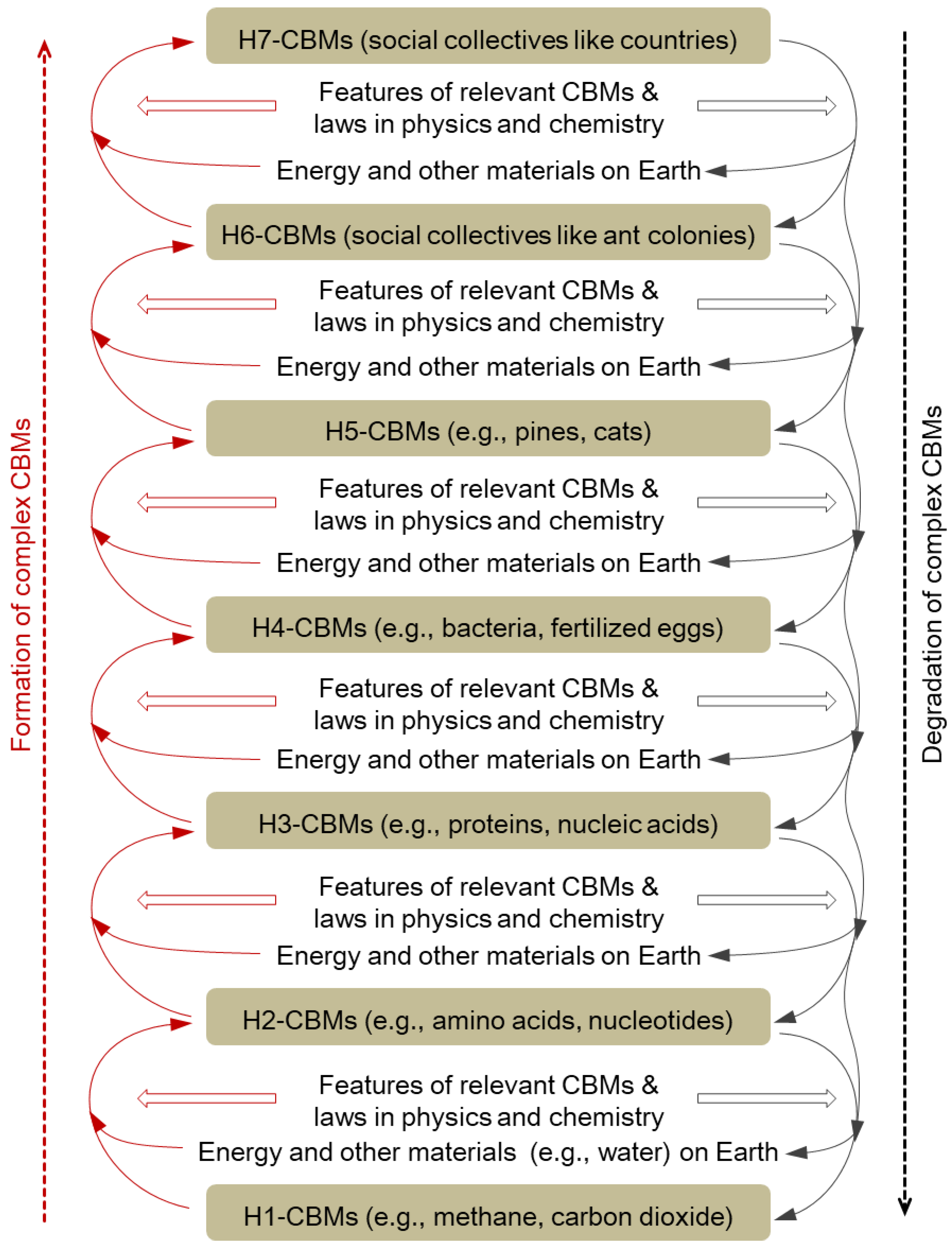
CBMs can undergo various types of changes upon absorbing energy, such as an increase in temperature and the formation of more complex CBMs (
Figure 1). Certain H1-CBMs, such as HCN, CO
2, and CH
4, along with other materials, form H2-CBMs, like amino acids, nucleotides, and monosaccharides, through some energy-absorbing organic synthesis reactions under the laws of thermodynamics and organic chemistry [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. Similarly, certain H2-CBMs, along with other materials, form H3-CBMs, like proteins, nucleic acids, and lipids, through some energy-absorbing organic synthesis reactions under the laws of thermodynamics and organic chemistry [
7,
8,
9]. Some H3-CBMs and other molecules form H4-CBMs (unicellular organisms like bacteria) through some energy-absorbing organic synthesis reactions and energy-consuming physical movements. Some H4-CBMs and other materials form H5-CBMs (multi-cellular organisms like birds and trees) through some energy-absorbing organic synthesis reactions and energy-consuming physical movements. For example, some fertilized eggs and related materials develop into adult elephants through many energy-absorbing organic synthesis reactions and many energy-consuming physical movements. Some animal individuals (e.g., ants and bees) form animal social collectives (H6-CBMs) with energy primarily derived from their food metabolism through organic decomposition reactions. Some human H6-CBMs form multi-hierarchy social collectives (H7-CBMs), such as universities and armies, with energy from food and other sources, such as electricity, fossil fuels, and wind energy.
Complex CBMs formed through the above energy-absorption processes are usually subject to degradation due to the sixth feature of CBMs listed in
Section 3.3. Complex CBMs can be established hierarchy by hierarchy and degraded into CBMs one or more hierarchies lower, and the degradation usually releases energy and some relatively simple CBMs and other materials (
Figure 1). The degraded complex CBMs and some other materials can form new complex CBMs through the above energy-absorption processes again. Regenerated complex CBMs usually carry some structural changes due to the seventh feature of CBMs listed in
Section 3.3. Therefore, there are cycles of formation and degradation of complex CBMs, and many complex CBMs carry some structural changes over these cycles. The cycles constitute a major part of the carbon cycle on Earth between the atmosphere, land, oceans, biosphere, and human society [
23,
25].
5. The Structure-Function Mechanism
It is a fact that some complex CBMs formed through the above energy-absorption processes possess new functions that less complex CBMs do not. For example, bird cells cannot fly, but birds can. This aligns with the core principle of systems theory: the whole is greater than the sum of its parts [
30].
It is also a fact that some structural variations of some complex CBMs can change the functions of the complex CBMs during the cycles of formation and degradation of complex CBMs (
Section 4). For example, genomic mutations can change the running rates of goats, communication within insect colonies, and the intelligence of humans.
The above two facts stem from the logic that different structures can serve different functions. They constitute the structure-function mechanisms of the evolution of CBMs and provide some new functions of complex CBMs that can facilitate or hinder the formation and accumulation of complex CBMs, as shown by the following examples.
(1) Some CBMs can catalyze some chemical reactions. For instance, various H2-CBMs (e.g., proline [
31]), H3-CBMs (e.g., many enzymes), and some CCMs between H2-CBMs and H3-CBMs (e.g., certain peptides [
32]) can catalyze the synthesis or degradation of certain H2-CBMs and H3-CBMs. These catalyzers facilitate the evolution of CCMs [
33].
(2) Some CBMs provide constituent materials for some other CBMs. For instance, amino acids, nucleotides, and monosaccharides can serve as “bricks” for the construction of proteins, nucleic acids, and polysaccharides, respectively.
(3) Some CBMs can capture constituent materials for themselves. For example, plants can capture CO2 from the air and water from their roots to synthesize glucose.
(4) Some CBMs provide energy. For instance, degradation of some H2-CBMs and H3-CBMs releases heat or energy.
(5) Some CBMs can actively absorb energy. For example, cows browse grasses which provide energy and constituent materials for cows.
(6) Some CBMs protect themselves or other CBMs. For instance, lipids shield H2-CBMs and H3-CBMs that are soluble in lipids from radiation and degradation enzymes, and liposomes safeguard H2-CBMs and H3-CBMs that are water-soluble from certain enzymes.
(7) Some CBMs provide direction of the precise synthesis of some H3-CBMs. For example, nucleic acids (DNA or RNA) can serve as the template to direct the synthesis of DNA, RNA, and proteins, ensuring their precise extension according to specific sequences.
(8) Some CBMs can reproduce. For example, H4-CBMs developed the ability to integrate the previous seven functions to replicate themselves.
(9) Some organisms have the sexual selection function. For example, female lions mate with male lions who have demonstrated their dominance through fighting.
(10) Some organisms have the non-random mutation function. For example, bacteria can reduce random mutations at important genomic sites, and human bodies enhance the mutation frequency in immune genes [
34,
35].
(11) Some organisms can encounter epigenetic changes. Some epigenetic changes in gene functions are heritable and can result in alterations to biological traits, despite the fact that the sequence of the relevant genes remains unchanged [
36].
(12) Humans can accumulate knowledge. This function is due to the high intelligence of humans, which stems from the intricate structure of their brains.
(13) Humans can manufacture silicon-based lives that can be extremely powerful in intelligence and longevity and can significantly shape the evolution of human societies and the environment.
6. The natural selection mechanism
As elucidated in
Section 4, there are cycles of formation and degradation of complex CBMs, and many complex CBMs carry some structural changes over these cycles. These cycles hold the following three mathematical logics.
Logic 1: If the total formation of a complex CBM exceeds its total degradation, this complex CBM persists on Earth.
Logic 2: If the ratio of the formation to degradation of a complex CBM exceeds 1 (or falls below 1), the quantity of this complex CBM is increasing (or decreasing) during the relevant period.
Logic 3: If the ratio of the formation to degradation of complex CBM A is greater than that of complex CBM B, the ratio of CBM A versus CBM B in quantity is increasing during the relevant period.
The above three mathematical logics constitute the natural selection mechanism, which has the following features:
(1) According to the CBET, natural selection stems from mathematical logics, while in previous theories, natural selection stems from survival competition of organisms. The mathematical logics are the essence of natural selection, and survival competition is the phenomenon of natural selection in the biosphere.
(2) Natural selection in the CBET applies to chemical evolution, biological evolution, and social evolution, while natural selection in previous theories is largely restricted to organisms or biological evolution.
(3) The overall performance of the formation and maintenance (OPFM) of a complex CBM determines its fate in natural selection according to the three mathematical logics, and the OPFM itself is fully determined not by a single component, a single trait, or a single hierarchy of the complex CBM, but by all components, all traits, and all hierarchies of the complex CBM [
37]. This suggests that all components, all traits, and all hierarchies of complex CBMs are under natural selection. Meanwhile, certain traits of complex CBMs, such as the running ability of antelopes, the group collaboration of wolves, and human individual expertise, can significantly enhance their OPFM. Therefore, natural selection highlights all-round development and specialized development.
(4) Natural selection in the CBET exhibits inclusiveness because, according to Logic 1 stated above, a complex CBM can persist on Earth if its rate of formation exceeds its rate of degradation, regardless of whether its OPFM is lower than that of its parents or other CBMs or whether it has some disadvantages. Natural selection allows organisms to have some disadvantages if their OPFM is sufficient. For example, many people carry gene mutations associated with genetic diseases. This further suggests that some biological traits (e.g., the long necks of giraffes) may be advantageous, neutral, or disadvantageous in natural selection. Additionally, the inclusiveness of natural selection is in line with the abundance of biological diversity and the abundance of neutral mutations in genomes, as these mutations have minimal impact on the OPFM of organisms [
17]. In contrast, previous theories emphasized the fierce competition inherent in natural selection, which was expressed as “survival of the fittest” in Darwin's theory and “gradual replacement of populations with those carrying advantageous mutations” in the Modern Synthesis [
17].
(5) Natural selection in the CBET can provide an interpretation for some macroevolution events. For example, the inclusiveness of natural selection in the CBET suggests that sympatric speciation could occur because different mutants with distinct trait combinations can all maintain sufficient OPFM within the same ecological niche and area, particularly when their populations are far from saturation in the area, like in scenarios of adaptive radiation [
38]. For example, antelopes and buffaloes have different traits to confront carnivores, which all provide them with sufficient OPFM in natural selection, and hence they could complete their speciation in the same ecological niche. Previously, it was assumed that sympatric speciation within the same ecological niche was impossible [
39]. Moreover, organisms, multicellular organisms, and endothermic animals, are not inherently fitter than inanimate materials, unicellular organisms, and ectothermic animals, respectively, yet they can all maintain sufficient OPFM (i.e., their formation exceeds their degradation) due to some functions realized by their complex structures, which allows them to persist and evolve. Therefore, the inclusiveness of natural selection and the aforementioned driving force mechanism and the structure-function mechanism can explain the origin and development of life, multicellular organisms, and endothermic animals.
(6) Natural selection in the CBET is influenced by both inheritable and non-inheritable factors. For example, some genetic factors can enhance the OPFM, while non-heritable factors such as education and vaccination can also increase the OPFM. This underscores innate advantages and acquired strengths. Additionally, epigenetic changes, whether heritable or not, can affect an organism's OPFM and, consequently, affect natural selection [
40].
(7) Natural selection in the CBET is influenced by the environment. Whether an HHCBM has sufficient OPFM depends not only on its own characteristics but also on the environment. Therefore, organisms must adapt to their environment or migrate to more suitable areas for survival and reproduction. Similarly, humans should work to protect the environment.
(8) Natural selection in the CBET involves competition, elimination, collaboration, and progressiveness, as suggested by Logic 2 and Logic 3 stated above. Competition results in the accumulation of beneficial changes (positive selection) and the elimination of detrimental changes (negative selection) in relation to the OPFM of complex CBMs. The combined impact of positive and negative selection is a continuous process of optimizing the inner structures, enhancing orderliness, and fostering collaboration within the relevant complex CBMs, which exhibits a progressive tendency of the evolution of CBMs. This aligns with the principles of Darwin's theory and the Modern Synthesis.
7. The evolution of CBMs from the lens of the CBET
7.1. The core viewpoints of the CBET
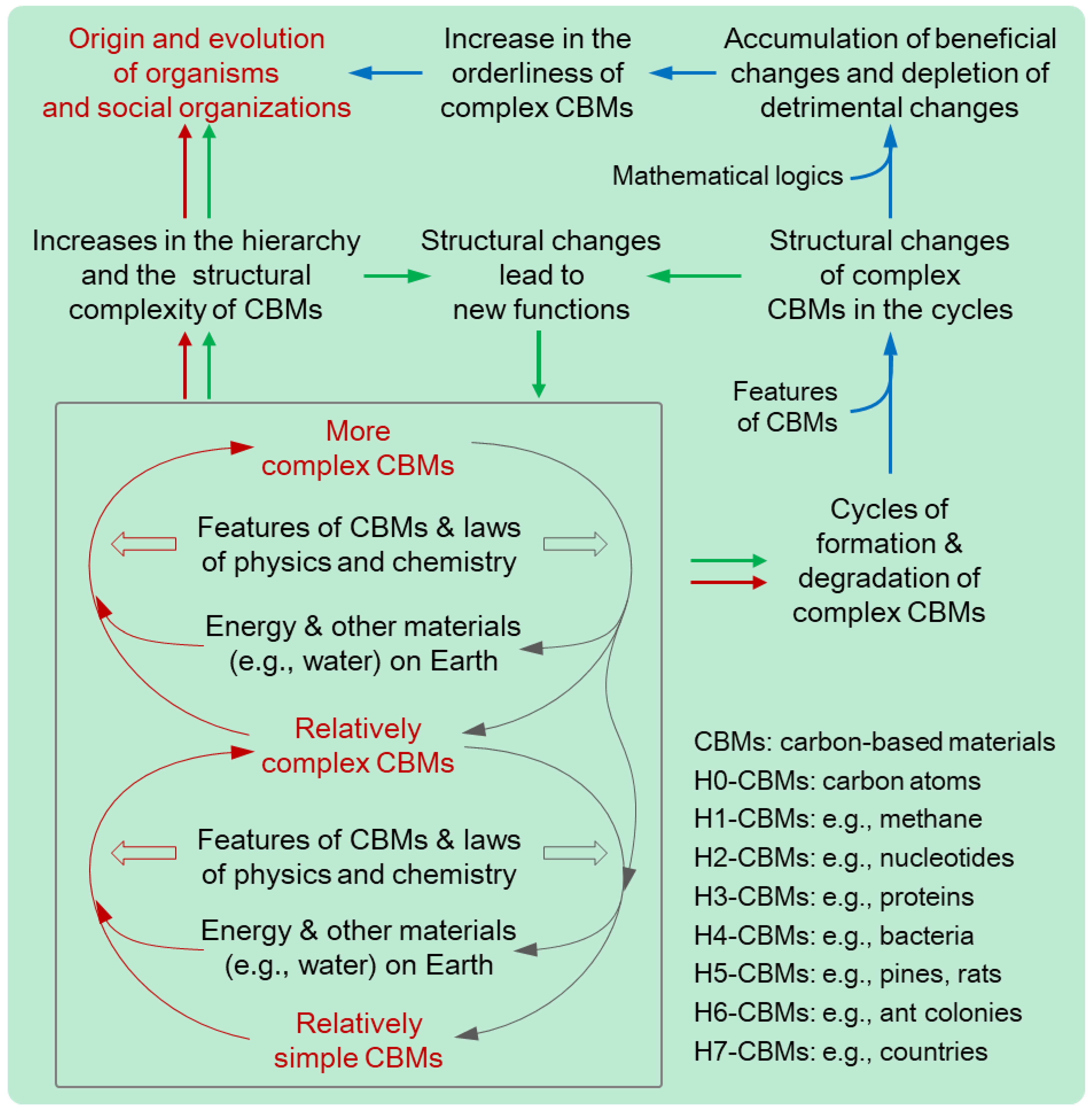
(1) Many substances on Earth spontaneously or actively absorb energy from some energy sources, such as sunlight, geothermal energy, cosmic radiation, water flow, wind, etc., on Earth, under some principles of classical physics and chemistry (e.g., laws of thermodynamics and organic chemistry). This constitutes the driving force mechanism that provides energy for the evolution of CBMs on Earth.
(2) Some relatively simple CBMs, along with some other materials, can form relatively complex CBMs after energy absorption, due to the features of the relevant CBMs. Some of these relatively complex CBMs, along with some other materials, can form more complex CBMs after energy absorption, due to the features of the relevant CBMs. The increase in the structural complexity of CBMs can provide complex CBMs with some new functions.
(3) Most complex CBMs formed through the above energy absorption processes will be degraded due to some features of CBMs, and the degraded complex CBMs can form complex CBMs again through the above energy absorption processes. The regenerated complex CBMs usually carry some structural variations due to some features of CBMs. Therefore, there are cycles of formation and degradation of complex CBMs with structural variations in complex CBMs. The structural variations can provide some complex CBMs with some new functions.
(4) Some complex CBMs can obtain new functions through the above two types of structural changes, which constitute the structure-function mechanism. This mechanism generates new functions for the evolution of CBMs on Earth. Some of the new functions, in turn, can aid the relevant CBMs or other CBMs to form more complex CBMs through energy absorption.
(5) In mathematics, the cycles of formation and degradation of complex CBMs with structural variations in complex CBMs lead to the accumulation of the variations beneficial to the formation and maintenance of complex CBMs and the depletion of detrimental variations, which constitutes the natural selection mechanism.
(6) The synergy of the above three mechanisms results in the progression from chemical to biological and social evolution, marked by the escalating hierarchy of CBMs and the increase in the quantity, diversity, and orderliness of high-hierarchy CBMs. The driving force mechanism and the structure-function mechanism co-determine that CBMs on Earth have the tendency to absorb more energy and more materials to form CBMs with more complex structures and higher hierarchies [
5,
41], while the nature selection mechanism determines that CBMs on Earth have the tendency to utilize the absorbed energy and materials to form and maintain complex CBMs in a more efficient way.
In one sentence, the CBET can be so expressed: Due to some principles of physics and chemistry, features of Earth, and features of CBMs, three mechanisms in nature underpin the evolution of CBMs with energy, functions, and orderliness, respectively, driving the progression from simple and unordered carbon-containing molecules to complex, orderly, and diverse organisms and social organizations (
Figure 2).
The evolution of CBMs has a significant impact on its own development, as it stores energy, prepares materials, develops catalysts, and generates novel functions to support the subsequent stages of the evolution of CBMs (
Figure 1 and
Figure 2). Additionally, the evolution of CBMs significantly alters Earth's surface and their environments, creating opportunities, fostering competition, and potentially leading to disasters for other CBMs [
42]. For example, the increase in photosynthetic bacteria round 2500 million years ago likely resulted in a significant increase in the concentration of oxygen in the air, which posed a disaster to anaerobic bacteria and opportunities for aerobic bacteria [
17,
43].
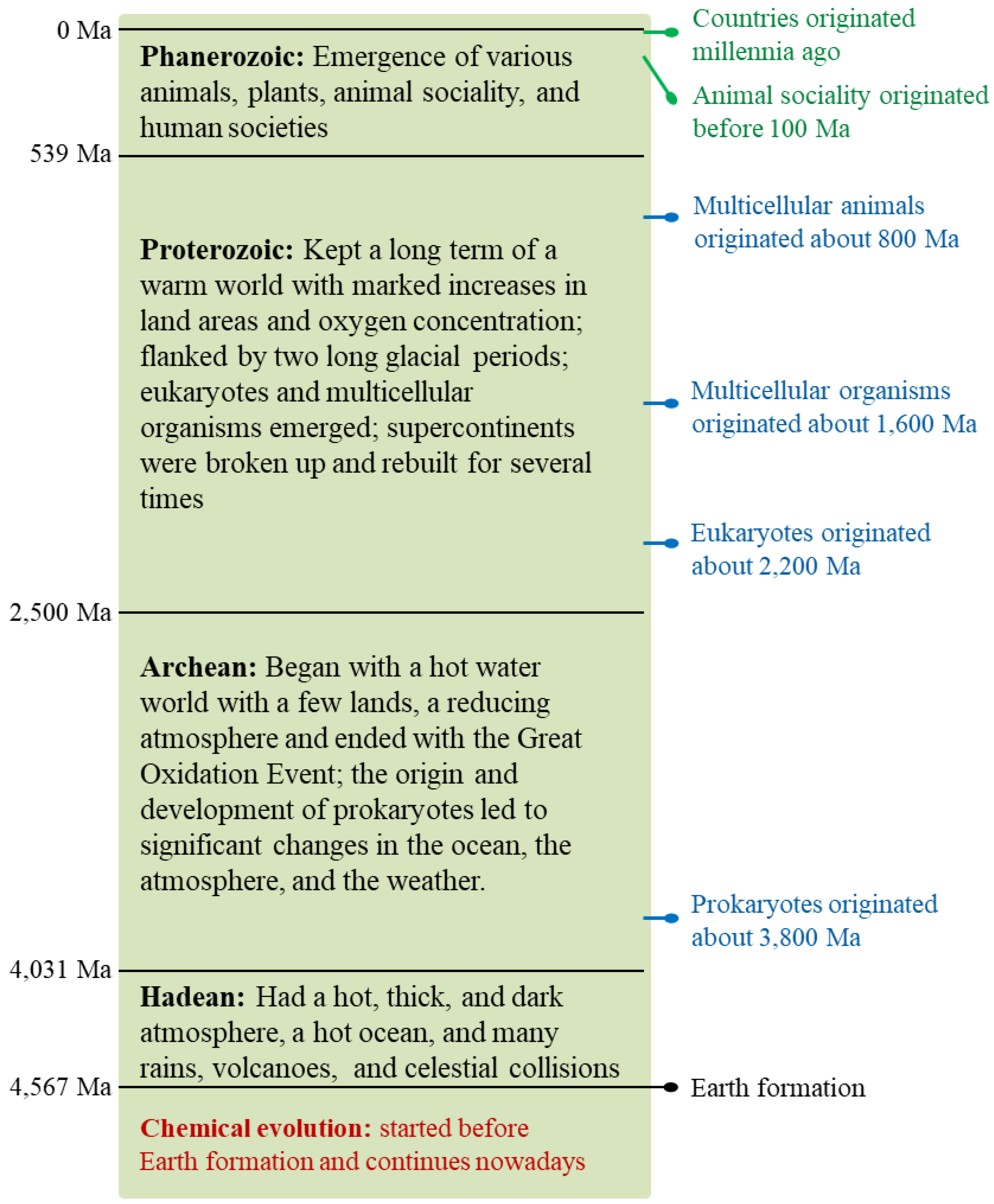
Earth formed 4.6 billion years ago. Its history has been divided into four eons: Hadean, Archean, Proterozoic, and Phanerozoic [
44] (
Figure 3), and it has experienced chemical evolution, biological evolution, and social evolution, as elucidated below in
Section 7.2−7.4.
7.2. Chemical Evolution from the Lens of the CBET
Chemical evolution began before the formation of Earth and continues to the present day. According to current cosmological understanding, H0-CBMs (carbon atoms) on Earth were formed within the interiors of giant or supergiant stars. These atoms were scattered into space as dust during the explosive deaths of these stars in the form of powerful and luminous supernovae. The dust from these supernovae events eventually coalesced to form the Sun, Earth, and other celestial bodies within our solar system [
24].
During and after the formation of Earth, H0-CBMs combined to form H1-CBMs (e.g., carbon dioxide, methane, and hydrogen cyanide). Through heat-absorbing chemical reactions, these H1-CBMs gave rise to a multitude of distinct H2-CBMs, a process that is widely accepted in modern science [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. The prebiotic chemical synthesis routes for various H2-CBMs found in organisms, such as amino acids, nucleotides, and monosaccharides, have been experimentally validated under geologically plausible and biologically relevant conditions in laboratories [
10,
11,
12,
13,
14,
15]. Moreover, myriad distinct H2-CBMs have been identified in meteorites, with mass spectrometry analysis of the Murchison meteorite, which fell in Australia in 1969, suggesting the presence of possibly millions of distinct CCMs [
45]. This evidence supports the hypothesis that myriad distinct H2-CBMs formed through heat-absorbing synthesis reactions prior to Earth's formation.
The prebiotic chemical synthesis routes for H3-CBMs, such as proteins, nucleic acids, lipids, and polysaccharides, which are essential in organisms, have been the subject of exploration for decades, resulting in significant advances. For instance, studies have shown that elements such as phosphorus, boron, and others can aid in the polymerization of proteins and nucleic acids in a prebiotic setting [
6,
7,
8].
The CBET posits that, as a result of the structure-function mechanism, there should have been an evolution of catalysts for the polymerization of H3-CBMs, from small organic or inorganic molecules to intermediate CCMs and then to large CCMs, with an increase in the efficiency and specificity of the catalysts as the structural complexity of the catalysts increased. Consequently, a multitude of distinct proteins, nucleic acids, and other CBMs could have accumulated before the origin of life.
Natural selection can enhance the efficiency of the chemical synthesis of H2-CBMs and H3-CBMs in the inanimate world and the biosphere. However, the total amount of H2-CBMs and H3-CBMs on Earth is influenced by multiple factors. For example, asteroid impacts, volcanic eruptions, glacial periods, and ecosystem destruction can all lead to mass extinctions of organisms and mass reductions in the populations of H2-CBMs and H3-CBMs [
17,
46].
7.3. Biological Evolution from the Lens of the CBET
The myriad distinct H2-CBMs and H3-CBMs that emerged on Earth before the origin of life could spontaneously form myriad multiple-molecule structures due to the actions of wind, water flow, evaporation, and other factors. Among these structures, some had one of the first seven functions listed in
Section 5, and a very few had all these seven functions, or the self-reproduction function, due to the complex collaboration of various molecules (Section 5.2). These rare CBMs constituted the first batch of H4-CBMs, which possibly originated at seabeds near hydrothermal vents in the ocean [
47]. One of them passed natural selection and became the Last Universal Common Ancestor (LUCA) of all living things. LUCA could have hundreds of genes [
48]. The possibility of the abiogenesis of H4-CBMs, including LUCA, has been supported by successful experiments regarding the synthesis of viruses and H4-CBMs [
49,
50]. However, the concrete process of the origin of LUCA, particularly the step about the origin of codons for protein synthesis [
51], remains unknown.
Billions of years after the origin of LUCA, myriad variants of H4-CBMs accumulated on Earth, and some of them became eukaryotes in the early Proterozoic eon [
52]. In the middle Proterozoic eon myriad, variants of eukaryotes accumulated on Earth, and some of them became multicellular organisms (H5-CBMs) [
53]. As elucidated in
Section 6, the origin of H4-CBMs, H5-CBMs, and various species (e.g., amphibians [
34]) was not because they were fitter than their previous taxa, but because they were fit in natural selection: they can obtain sufficient materials and heat or energy to reproduce and maintain themselves due to their complex structures, although they are vulnerable compared to many inanimate materials.
Some features of biological evolution, like that natural selection of organisms is both competitive and inclusive, have been clarified in
Section 5 and
Section 6.
Among the thousands of catalytic molecules in an organism, only a few molecules, such as ribozymes, thrombin, and hammerhead ribozymes, are autocatalyzers that catalyze a step in their own production process. Moreover, no large CCMs have been found that can catalyze all steps of their own synthesis. For example, ribozymes catalyze the formation of ribozymes, but they only catalyze the incorrect folding of ribozymes and cannot catalyze the many steps in the synthesis of ribozymes from certain amino acids in a specific sequence. In biology, the reproductive function of H4-CBMs comes from the collaboration of various molecules and many catalytic molecules that do not catalyze their own synthesis (termed allocatalyzers in this article). Therefore, H4-CBMs are not the hyper-cycle systems composed of autocatalytic molecules hypothesized by Manfred Eigen in the 1970s [
16], but the hyper-cycle systems formed by the collaboration of various allocatalytic molecules with many other molecules, including those providing energy, guiding the precise synthesis of specific molecules, and protecting these molecules. The complex cooperative relationships between these molecules achieve the first seven functions listed above in
Section 5, and these functions collectively achieve the function of reproducing offspring of H4-CBMs. The organic large molecules hypothesized by Manfred Eigen to exist in the hyper-cycle system with both autocatalytic and allocatalytic effects, capable of undergoing mutations, have not yet been discovered in the world. For the same reasons, the RNA world hypothesis, which overestimated the incomplete autocatalytic property of RNA and overlooked the collaboration of various molecules in the origin of life [
54], is questionable.
7.4. Social Evolution from the Lens of the CBET
Animal or human social collectives (H6-CBMs and H7-CBMs) have the features of the collaboration of animal or human individuals with different duties working for the collectives. Such closely collaborative collectives can be viewed as superorganisms, while multicellular organisms can be viewed as social collectives of cells, and unicellular organisms as social collectives of molecules.
Fossils and molecular clocks both suggested that multicellular animals possibly emerged on Earth 800 million years ago [
55]. Animals actively search for and consume food, which provides them with constituent materials and heat or energy. Possibly 100 million years ago, some insects established their social collectives [
56,
57]. The increased complexity in gene regulation and chemical communication is important to the origin of sociality in insects [
56,
57,
58], which coincides with the structure-function mechanism of the CBET.
Sociality has been established in around 24,000 species of insects and some species of crustaceans and mammals, which constitute separate events in social evolution [
56,
57]. Sociality is widespread in
Hymenoptera (ants, bees, and wasps) and
Blattodea (termites). A typical social collective has a queen and a few reproductive males, who take on the roles of the sole reproducers, and other individuals act as soldiers and workers who work collaboratively to create a living situation favorable for the brood.
Animal social collectives have complex functions stemming from their complex structures. They significantly reduce intra-population competition and struggles, as well as utilize collective advantages to obtain relevant materials and energy to reproduce and maintain them and confront natural selection. Consequently, although sometimes social animals require individuals to sacrifice their freedom and even their lives for the benefit of others, social animals have strong natural selection advantages due to the collaboration within animal social collectives, and they typically have significantly longer lifespans compared to their counterparts without sociality within the same taxa [
56,
57,
58,
59]. For example, the naked mole rat (
Heterocephalus glaber) living in society has a lifespan of up to 30 years, several times longer than that of other rodents. Moreover, they reproduce themselves through the reproduction of a few strong individuals and cooperative brood care, and this specialized breeding can easily accumulate beneficial changes and eliminate harmful ones. On the other hand, the natural selection advantages of animal social collectives also lead to sometimes intense competition or conflicts between animal social collectives. For instance, battles between ant colonies often result in the slaughter of numerous ants [
60].
H6-CBMs have a single management hierarchy. For example, no ant controls multiple colonies of ants in the same location.
Due to the complex brains, bipedal bodies, unique vocal cords, and other special structures of humans, humans possess high intelligence, language and written communication, knowledge accumulation, and collaboration capabilities. Building upon these advantages, humans have established universities, armies, banks, countries, and various other social collectives (H7-CBMs) that have multiple hierarchies in management.
In H7-CBMs, low-hierarchy social collectives adhere to the laws of physics, chemistry, and biology, as well as the rules of social collectives, serving high-hierarchy social collectives. High-hierarchy social collectives benefit from the collaboration between low-hierarchy social collectives, enabling them to acquire resources, defend themselves, reproduce, and decrease internal competition and conflicts more efficiently. Therefore, although sometimes human collectives may need to sacrifice individual freedom or even the lives of certain individuals (such as soldiers, policemen, or firefighters) to protect social collectives and other individuals, humans generally have longer lifespans compared to other primates.
The natural selection advantages of human social collectives usually increase as the hierarchy of the collective rises. This is why most humans worldwide have established multi-hierarchy social collectives largely along the line from clans to tribes, tribal alliances, nations, and national alliances. However, the natural selection advantages of human social collectives can also intensify competition and conflicts between high-hierarchy human societies, including wars. In 2022, global military spending reached
$2.2 trillion, and 238,000 people died as a result of wars [
61]. Advances in technology, such as the development of nuclear weapons and artificial intelligence, have significantly augmented the destructive potential of international conflicts, posing a threat to humanity and Earth. These enormous military expenditures and casualties, as well as the threats to humanity and Earth could be circumvented if a global and harmonious human social collective were established, like the fact that in a harmonious country, there are no economic losses or human deaths due to internal wars. Therefore, the CBET asserts that one of the rational ultimate objectives of the evolution of CBMs is to unite all countries into a single harmonious social collective, which, in essence, requires that the rational interests of most human individuals and human social collectives be respected in social rules and management. Accordingly, the CBET advocates for the peaceful and harmonious development of human society.
7.5. The Natural Roots of Multiple Important Social Notions
From the view of the CBET, the natural roots of the social notions of all-round development, specialized development, inclusiveness, competition, elimination, collaboration, inherited advantages, acquired strengths, diversified development, and harmonious and peaceful development of human society have been clarified in
Section 6 or
Section 7.4. The natural roots of other social notions, such as selfishness, altruism, restriction of freedom, and freedom, are elucidated below.
In the survival competition of natural selection, selfishness is essential for myriad animals to obtain adequate constituent materials, energy, suitable environments, and mating opportunities to maintain their lives and reproduce themselves. Meanwhile, altruism is also widespread throughout the evolution of CBMs: many molecules are the catalyzers, energy-providers, or constituent materials for the synthesis of other molecules, including H2-CBMs and H3-CBMs; many molecules in H4-CBMs (e.g., bacteria) help the passage of nucleic acids to the next generation; many cells in H5-CBMs (e.g., humans) help the passage of reproductive cells to the next generation; many animal individuals in H6-CBMs (e.g., ant colonies) help the reproduction of a few reproductive individuals in the collectives; and many human individuals in H7-CBMs (e.g., soldiers, policemen, and firefighters in a country) sacrifice themselves for the benefit of the whole collectives or other individuals.
Restriction of the freedom of LHCBMs in HHCBMs is also widespread because HHCBMs are established on the collaboration of their inner LHCBMs, which requires the LHCBMs to sacrifice their freedom for the collaboration. However, the freedom of LHCBMs increases with the increase in the hierarchies of CBMs. For example, many atoms can hardly conduct relative motion inside H1-CBMs and H2-CBMs, but they can conduct nanometer-sized relative motion in H3-CBMs. Many molecules can conduct micrometer-sized relative motion inside cells. Many cells can conduct meter–sized movement in multicellular organisms, and many animal individuals in animal groups can move relatively freely in certain areas. As for many animals, freedom is important for them to search for and consume sufficient food, fight against predators, obtain mating opportunities, and reproduce themselves. Furthermore, freedom is important for animals to move to more suitable environments.
8. Novelties of the CBET
In science, the CBET elucidates that the evolution of CBMs arises from the synergy of three mechanisms, which provide the evolution of CBMs with energy, functions, and orderliness, respectively. The CBET thus answers the core science question of why and how inanimate matter evolved into complex organisms and societies in a novel, explicit, and relatively comprehensive way. In contrast, the three phases of the evolution of CBMs, namely chemical evolution, biological evolution, and social evolution, were usually investigated separately in previous theories, and few theories have explicitly interpreted the mechanisms of the evolution of CBMs from a panoramic view, although some significant efforts on this topic have been published [
5,
42,
62]. Moreover, only one mechanism, such as natural selection in Darwin's theory [
17], entropy dissipation into the surroundings in Schrödinger's negative entropy notion [
3], self-organization in Prigogine's dissipative structure theory [
4], the constructal law [
62], the maximum entropy production principle [
5,
63], or the free-energy principle [
64], was proposed to explain the evolution of CBMs in previous theories or hypotheses. These one-mechanism theories cannot explicitly or comprehensively explain the evolution of CBMs. Moreover, they overlooked or even rejected the crucial role of natural selection, except for Darwin's theory and the Modern Synthesis. Although Darwin's theory and the Modern Synthesis highlighted the importance of survival selection and natural selection, they did not clarify why and how organisms can exist. They assumed that natural selection, gene drift, competition, or mutations are the driving forces of evolution [
65,
66,
67,
68,
69], but these factors require energy and do not provide energy for the evolution of CBMs. This is analogous to the fact that an engine and cycles are essential for a fuel-powered car to run, but the engine and the cycles cannot provide the driving force for the car. It is fuel that provides the driving force.
In physics, the CBET challenges some widely accepted viewpoints regarding thermodynamics and evolution. The second law of thermodynamics, which states that heat can spontaneously flow from hotter to colder objects and not the reverse, can also be mathematically expressed as the entropy of isolated systems increasing over time. Since entropy is commonly assumed to be a measure of disorder and the entire universe can be considered as an isolated system, the law indicates that the universe tends to become more and more disordered, which contradicts evolution, a natural process characterized by an increase in orderliness [
3,
4,
63,
64,
70]. Creationists (including some scientists) have exploited this perceived contradiction to argue for the existence of divine entities [
2]. Some influential theories, such as Schrödinger's negative entropy theory and Prigogine's dissipative structure theory, have attempted to reconcile this contradiction by suggesting that open systems (like organisms) can gain orderliness through the dissipation of entropy into their environment. [
3,
4]. These theories accepted the notion that entropy represents disorder and overlooked the important roles of energy, chemical reactions, the special features of carbon atoms and other CBMs, and natural selection. In contrast, the CBET embraces the new notion that entropy and disorder have distinct meanings, and hence entropy cannot represent disorder [
41,
71], so the second law of thermodynamics does not contradict evolution. Furthermore, the CBET clarifies that the second law of thermodynamics is highly associated with the driving force of evolution (
Section 4), which coincides with some research [
5,
62,
70]. Additionally, the complex relationships among the five basic concepts associated with evolution: materials, energy, structures, functions, and orderliness, which were frequently investigated with some notions associated with the elusive concept of entropy, are explicitly elucidated with the three mechanisms revealed by the CBET (
Figure 2).
In biology, as elucidated in
Section 6, the CBET reveals the driving force of biological evolution and the mathematical essence of natural selection and provides more comprehensive explanations regarding the targets of natural selection, biological altruism, biological diversity, and some macroevolution issues, such as the origin of life, multicellular organisms, endothermic animals, and sympatric speciation. Moreover, natural selection, non-random mutations, neutral mutations, epigenetic changes, and acquired strengths, which cannot be integrated into any previous evolutionary theory, are integrated into the cohesive framework of the CBET, as elucidated in
Section 5 and
Section 6.
In the social sciences, the CBET reveals the natural roots of multiple pivotal and seemingly paradoxical social notions, such as inclusiveness versus elimination, collaboration versus competition, altruism versus selfishness, and freedom versus restriction. Therefore, the CBET advocates for the balanced development of human society. For instance, cruel exploitation of the people or excessive welfare policies are both unreasonable. It is also unreasonable to prohibit citizens from traveling and to allow criminals to escape from prison. Throughout human history, countries that better implement the concept of balanced development in their social rules and management have often proven to be more competitive in international competitions. The CBET also elucidates the imperative of the integration of all countries into a single harmonious social collective. In contrast, previous evolutionary theories highlight selfishness, competition, and the elimination of those less advantageous in certain traits [
1]. These biased notions have historically been employed to rationalize colonialism, slavery, racism, and genocide [
18].
Together, the CBET provides better explanations for some evolutionary issues compared to previous theories (
Table 1).
9. Reliability of the CBET
(1) The factors highlighted in the CBET, such as the features of Earth, the features of carbon atoms and other CBMs, some principles of classical physics and chemistry (which apply to the hierarchies from atoms to the solar system where the evolution of CBMs occurred), energy, chemical reactions, new functions stemming from the changes in the structures of CBMs, and natural selection, are all critical for the evolution of CBMs. In contrast, previous theories only addressed a portion of these critical factors.
(2) The above factors critical to the evolution of CBMs are explicitly integrated in the CBET with the support of logics and evidence. The CBET, as elucidated in
Section 3, aligns with many facts in physics, chemistry, biology, geology, astronomy, and social sciences, and does not contradict any facts in physics, chemistry, biology, geology, astronomy, and social sciences. Moreover, the CBET does not harbor any weird viewpoints, elusive concepts, or complex mathematical formulas (
Figure 2), and the CBET is established on well-established knowledge in science, rather than on any novel laws hypotheses, observations, or experiments.
(3) As shown in
Section 8 and
Table 1, the CBET provides better explanations for multiple evolutionary issues and accommodates multiple evolutionary facts that cannot be integrated into any previous theories. This also supports the reliability of the CBET.
10. Conclusions and perspectives
This article creates the concepts of eight hierarchies of CBMs and establishes the CBET, which provides novel, explicit, and relatively comprehensive explanations for the evolution of CBMs on Earth from simple to complex and from unordered to ordered. This theory elucidates three key mechanisms shared by chemical evolution, biological evolution, and social evolution. It demonstrates the absence of conflict between the second law of thermodynamics and evolution, thereby refuting a key argument used by creationism and unifying biology with physics and chemistry. It reveals the mathematical essence of natural selection and provides better explanations for natural selection and some other evolutionary issues. The theory uncovers the underlying natural roots of multiple crucial social notions, such as inclusiveness, altruism, collaboration, and freedom, besides selfishness, elimination, competition, and restriction. Therefore, the CBET could be a basic theory spanning the natural sciences and social sciences.
The CBET can guide natural scientific research. For instance, on chemical evolution and the origin of life, this theory suggests searching for some CCMs (e.g., peptides) that can effectively catalyze the synthesis of proteins, nucleic acids, or other organic macromolecules associated with life. It also suggests investigating collaborative relationships among various molecules in the context of life's origin. It recommends a panoramic perspective for research on natural selection, macroevolution, and social behaviors in animals. However, the CBET cannot provide details for each event of the evolution of CBMs during the past billions of years, which should be investigated using some approaches from physics, chemistry, biology, geology, or the social sciences.
The CBET can guide social scientific research and social development because it uncovers the underlying natural roots of multiple crucial social notions and advocates for the balanced, harmonious, and peaceful development of human society, as well as the integration of all countries into a single harmonious social collective.
Acknowledgments
The authors thank Meng Yang for her various constructive comments. The authors thank Yiqing Chen for her contribution to Section 1, Section 6, and Section 7 as well as the figures. This work was supported by the High-Level Talent Fund of Foshan University (No. 20210036).
Conflictsof Interest
The authors declare no competing interests.
References
- Xie, P. The aufhebung and breakthrough of the theories on the origin and evolution of life (Science Press, 2014).
- Schreiber, A. , & Gimbel, S. Evolution and the second law of thermodynamics: Effectively communicating to non-technicians. Evo Edu Outreach, 2010. [Google Scholar] [CrossRef]
- Schrodinger, E. What is life (Cambridge University Press, 2012).
- Prigogine, I. Time, structure and fluctuation (Nobel Lecture). Science, 1978. [Google Scholar] [CrossRef]
- Dewar, R.C. Maximum entropy production and plant optimization theories. Philos Trans R Soc Lond B Biol Sci. 1429. [Google Scholar] [CrossRef]
- Oparin, A. I. Chemistry and the origin of life. R. Inst. Chem. Rev. 1969. [Google Scholar]
- Guo, X. , Fu, S., Ying, J., & Zhao, Y. Prebiotic chemistry: a review of nucleoside phosphorylation and polymerization. Open Biol. 2023. [Google Scholar] [CrossRef]
- Sumie, Y. , Sato, K., Kakegawa, T., & Furukawa, Y. Boron-assisted abiotic polypeptide synthesis. Commun. Chem. [CrossRef]
- de Graaf, R. , De Decker, Y., Sojo, V., & Hudson, R. Quantifying catalysis at the origin of life. Chemistry, 2023. [Google Scholar] [CrossRef]
- Nogal, N. , Sanz-Sánchez, M., Vela-Gallego, S., Ruiz-Mirazo, K., & de la Escosura, A. The protometabolic nature of prebiotic chemistry. Chem Soc Rev. 2023. [Google Scholar] [CrossRef]
- Chieffo, C. , Shvetsova, A., Skorda, F., Lopez, A., & Fiore, M. The origin and early evolution of life: Homochirality emergence in prebiotic environments. Astrobiology, 2023. [Google Scholar] [CrossRef]
- Fiore, M. Prebiotic chemistry and life’s origin (Royal Society of Chemistry, 2022). 2022. [Google Scholar] [CrossRef]
- Anna Neubeck, A. , & McMahon, S. Prebiotic chemistry and the origin of life, 2021. [Google Scholar] [CrossRef]
- Farías-Rico, J. A. , & Mourra-Díaz, C.M. A short tale of the origin of proteins and ribosome evolution. Microorganisms. [CrossRef]
- Ershov, B. Natural radioactivity and chemical evolution on the early Earth: Prebiotic chemistry and oxygenation. Molecules, 2022. [Google Scholar] [CrossRef]
- Eigen, M. , & Schuster, P. Stages of emerging life — Five principles of early organization. J. Mol. Evo. 1982. [Google Scholar] [CrossRef]
- Futuyma, D.J. , & Kirkpatrick, M. Evolution, 2017. [Google Scholar]
- Rudman, L.A. , & Saud, L.H. Justifying social inequalities: The role of social Darwinism. Pers. Soc. 1155. [Google Scholar] [CrossRef]
- Richerson, P.J. , & Christiansen, M.H. Cultural evolution: Society, technology, language, and religion, 2013. [Google Scholar]
- Laland, K.N. Darwin's unfinished symphony: How culture made the human mind (Princeton University Press, 2017).
- Seager, S. Exoplanet habitability. Science, 2013. [Google Scholar] [CrossRef]
- Charette, M.A. , & Smith, W.H.F. The volume of Earth's ocean. Oceanography, 2010. [Google Scholar] [CrossRef]
- Carbon (https://en.wikipedia.org/wiki/carbon, accessed 9 April 2024).
- Roston, E. The carbon age: How life's core element has become civilization's greatest threat (Walker & Company, 2008).
- Li, Z.H. , Bailleul, A. M., Stidham, T. A., Wang, M., & Teng, T. Exceptional preservation of an extinct ostrich from the Late Miocene Linxia Basin of China. Vertebrata PalAsiatica 59, 229 (2021). Li, Z.H., Bailleul, A. M., Stidham, T. A., Wang, M., & Teng, T. Exceptional preservation of an extinct ostrich from the Late Miocene Linxia Basin of China. Vertebrata PalAsiatica. [CrossRef]
- Heck, P.R. , Greer, J., Kööp, L., et al. Lifetimes of interstellar dust from cosmic ray exposure ages of presolar silicon carbide. Proc. Natl. Acad. Sci. U. S. A. 1889. [Google Scholar] [CrossRef]
- Borgnakke, C. , & Sonntag, R.E. Fundamentals of thermodynamics.
- <i>Thermodynamics and chemistry </i>https://www2.chem.umd.edu/thermobook/v10-screen.pdf (DeVoe, H. 28. Thermodynamics and chemistry, 9 April.
- Martin, W.F. , Sousa, F.L., & Lane, N. Energy at life's origin. Science, 2014. [Google Scholar] [CrossRef]
- von Bertalanffy, L. General system theory: Foundations, development, applications (George Braziller, 1968).
- Morrell, D.G. Catalysis of organic reactions (CRC Press, 2019).
- Stone, E.A. , Cutrona, K.J., & Miller, S.J. Asymmetric catalysis upon helically chiral loratadine analogues unveils enantiomer-dependent antihistamine activity. J. Am. Chem. Soc. 1269. [Google Scholar] [CrossRef]
- de Graaf, R. , De Decker, Y., Sojo, V., & Hudson, R. Quantifying catalysis at the origin of life. Chemistry. 29, e202301447 (2023). [CrossRef]
- Fitzgerald, D.M. , & Rosenberg, S.M. What is mutation? A chapter in the series: How microbes “jeopardize” the modern synthesis. PLoS Genet. 2019. [Google Scholar] [CrossRef]
- Olivieri, D.N. , Mirete-Bachiller, S., & Gambón-Deza, F. Insights into the evolution of IG genes in amphibians and reptiles. Dev. Comp. Immunol. 2021. [Google Scholar] [CrossRef]
- Sabarís, G. , Fitz-James, M. H., & Cavalli, G. Epigenetic inheritance in adaptive evolution. Ann. N. Y. Acad. Sci. 2023. [Google Scholar] [CrossRef]
- Chen, J. , & Sun, Y. Variation in the analysis of positively selected sites using nonsynonymous/synonymous rate ratios: An example using influenza virus. PLoS One, 2011. [Google Scholar] [CrossRef]
- McGee, M.D. , Borstein, S.R., Meier, J.I., et al. The ecological and genomic basis of explosive adaptive radiation. Nature, 2020. [Google Scholar] [CrossRef]
- Mayr, E. Ecological factors in speciation. Evolution. 1947. [Google Scholar]
- Gómez-Schiavon, M. , Buchler, N.E. Epigenetic switching as a strategy for quick adaptation while attenuating biochemical noise. PLoS Comput Biol. 0736. [Google Scholar]
- Chen, J.M. , & Chen, J.W. Root of science—the driving force and mechanisms of the extensive evolution (Science Press, 2000), 2000. [Google Scholar]
- Benton, M.J. The red queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science, 2009. [Google Scholar] [CrossRef]
- Cohen, K.M. , Harper, D.A.T., Gibbard, P.L., & Car, N. International chronostratigraphic chart (https://stratigraphy.org/ICSchart/ChronostratChart2023-09.pdf, accessed 9 April 2024).
- Olejarz, J. , Iwasa, Y., Knoll, A.H., et al. The Great Oxygenation Event as a consequence of ecological dynamics modulated by planetary change. Nat. Commun. 2021. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P. , Gabelica, Z., Gougeonm R.D., et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. U. S. A. 2768. [Google Scholar] [CrossRef]
- Percival, L.M.E. , Ruhl, M., Hesselbo, S.P., et al. Mercury evidence for pulsed volcanism during the end-Triassic mass extinction. Proc. Natl. Acad. Sci. U. S. A. 7934. [Google Scholar] [CrossRef]
- 47. Dodd, M,S., Papineau, D., Grenne, T., et al. Evidence for early life in earth's oldest hydrothermal vent precipitates. Nature. [CrossRef]
- Weiss, M.C. , Sousa, F.L., Mrnjavac, N., et al. The physiology and habitat of the last universal common ancestor. Nat. Microb. 2016. [Google Scholar] [CrossRef]
- Stobart, C.C. , & Moore, M,L. RNA virus reverse genetics and vaccine design. Viruses, 2014. [Google Scholar] [CrossRef]
- Hutchison, C.A. 3rd, Chuang, R.Y., Noskov, V.N., et al. Design and synthesis of a minimal bacterial genome. Science, 6253. [Google Scholar] [CrossRef]
- Xie, P. Who is the missing "matchmaker" between proteins and nucleic acids? Innovation (Camb), 0012. [Google Scholar] [CrossRef]
- Zhu, S. , Zhu, M., Knoll, A. et al. Decimetre-scale multicellular eukaryotes from the 1.56-billion-year-old Gaoyuzhuang formation in North China. Nat. Commun. 2016. [Google Scholar] [CrossRef]
- Han, T.M. , & Runnegar, B. Megascopic eukaryotic algae from the 2.1-billion-year-old negaunee iron-formation, Michigan. Science, 1992. [Google Scholar] [CrossRef]
- Robertson, M.P. , & Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. 2012. [Google Scholar] [CrossRef]
- Anderson, R. P. , Woltz, C. R., Tosca, N. J., Porter, S. M., & Briggs, D. E. G. Fossilisation processes and our reading of animal antiquity. Trends Ecol Evol. 2023. [Google Scholar] [CrossRef]
- Zhao, Z. , Yin, X., Shih, C., Gao, T., & Ren, D. Termite colonies from mid-Cretaceous Myanmar demonstrate their early eusocial lifestyle in damp wood. Natl. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Mera-Rodríguez, D. , Jourdan, H., Ward, P.S., Shattuck, S., Cover, S.P., Wilson, E.O., & Rabeling, C. Biogeography and evolution of social parasitism in Australian Myrmecia bulldog ants revealed by phylogenomics. Mol. Phyogenet. Evol. 2023. [Google Scholar] [CrossRef]
- Nowak, M. , Tarnita, C., & Wilson, E. The evolution of eusociality. Nature, 2010. [Google Scholar] [CrossRef]
- Plowers, N. An introduction to eusociality. Nature Education Knowledge.
- Moffett, M.W. Adventures among ants (University of California Press, 2010).
- 61. SIPRI Military Expenditure Database (https://www.sipri.org/databases/milex, accessed 9 April 2024), 9 April.
- Bejan, A. The principle underlying all evolution, biological, geophysical, social and technological. Philos. Trans. A. Math. Phys. Eng. Sci. 2022. [Google Scholar] [CrossRef]
- Ramstead, M.J.D. , Badcock. P.B., & Friston, K.J. Answering Schrödinger's question: A free-energy formulation. Phys. Life Rev. [CrossRef]
- Skene, K.R. Systems theory, thermodynamics and life: Integrated thinking across ecology, organization and biological evolution. Biosystems, 5123. [Google Scholar] [CrossRef]
- Rott, P. , Grinstead, S., Dallot, S., et al. Genetic diversity, evolution, and diagnosis of sugarcane yellow leaf virus from 19 sugarcane-producing locations worldwide. Plant Dis, 3437. [Google Scholar] [CrossRef]
- Li, Z. , Liu, X., Wang, C., et al. The pig pangenome provides insights into the roles of coding structural variations in genetic diversity and adaptation. Genome Res. 1847. [Google Scholar] [CrossRef]
- Tang, R. , Zhu, Y., Yang, S., et al. Genome-wide identification and analysis of WRKY gene family in Melastoma dodecandrum. Int. J. 1490. [Google Scholar] [CrossRef]
- Wang, Y. , Li, X., Feng, Y. Autotetraploid origin of Chinese cherry revealed by chromosomal karyotype and in situ hybridization of seedling progenies. Plants (Basel), 2023. [Google Scholar] [CrossRef]
- Ma, S. , Guo, Y., Liu, D., et al. Genome-wide analysis of the membrane attack complex and perforin genes and their expression pattern under stress in the Solanaceae. Int. J. 2023. [Google Scholar] [CrossRef]
- Whitfield, J. Survival of the likeliest? PLoS Biol. 2007. [Google Scholar] [CrossRef]
- Chen, J.M. , & Chen, J.W. Disproving two widely accepted notions regarding entropy. Preprints, 2024. https://www.preprints.org/manuscript/202404.0655/v3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).