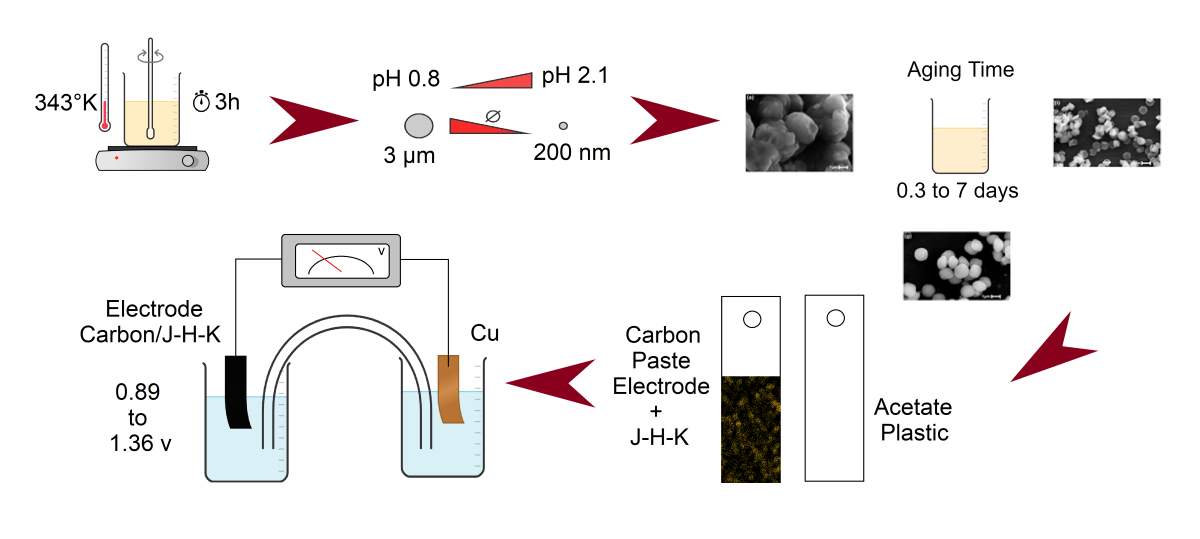
Structural and morphological properties of the hydronium-potassium jarosite microstructures were investigated in this work, and their electrical properties were evaluated. All microstructures were synthesized at a reasonable temperature of 343 K with a reduced reaction time of 3 hours. Increase in the pH from 0.8 to 2.1 decreased the particle sized from 3 µm to 200 nm and increasing the aging time from 0, 3 to 7 days resulted in semispherical, spherical and euhedreal jarosite structures, respectively. A Rietveld analysis also was done, finding that increasing pH, the amount of hydronium substitution by potassium in the cationic site also increases, having a 77.72 % of hydronium jarosite (JH) plus 22.29 % potassium jarosite (JK) at pH 0.8; 82.44 % (JH) and 17.56 % (JK) at pH 1.1, and 89.98 % (JH) plus 10.02 % (JK) at pH 2.1. The results obtained in this work show that the obtained hydronium potassium jarosite microstructures with reduced particle size and euhedreal morphology can be used as anode materials for improving the life time of lithium ion batteries, due that during the analysis of the voltage obtained using electrodes made with this particles and graphite, this ranged from 0.89 to 1.36 V.