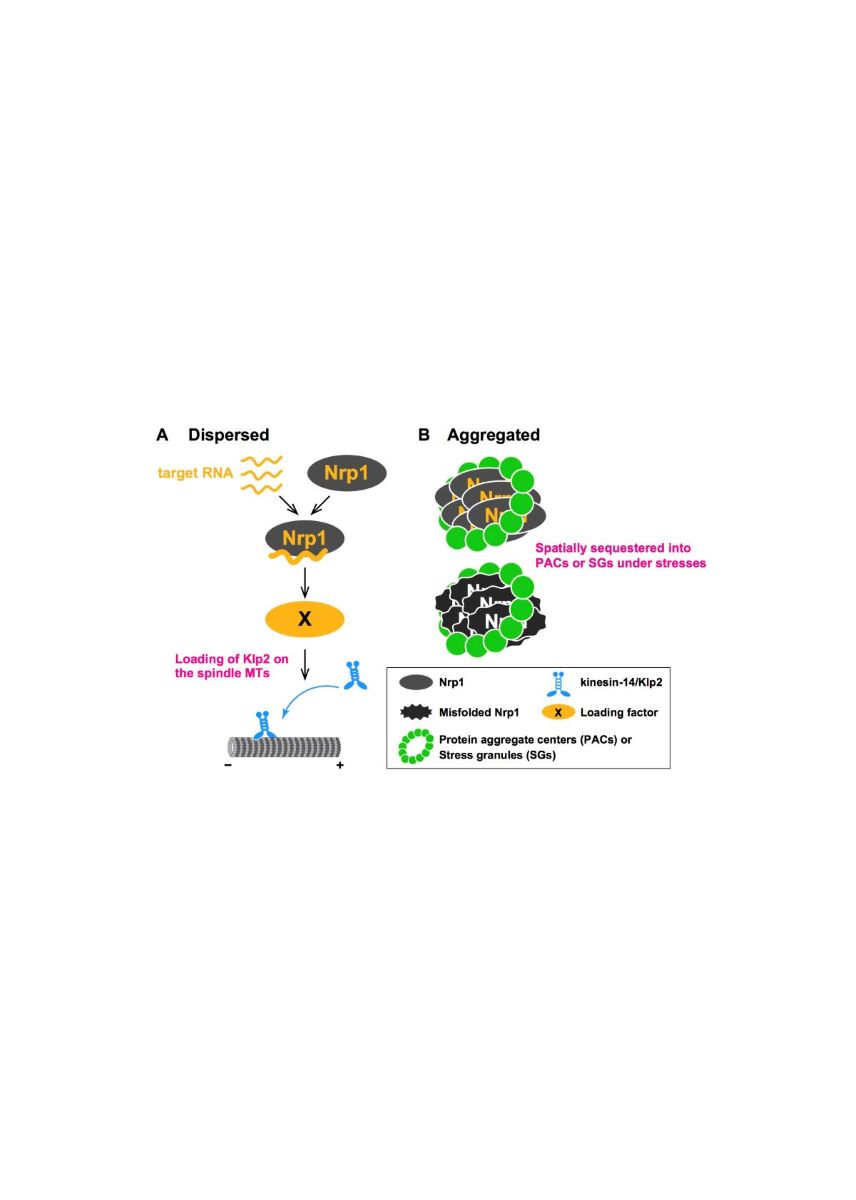
Cells form a bipolar spindle during mitosis to ensure accurate chromosome segregation. Proper spindle architecture is established by a set of kinesin motors and microtubule-associated proteins. In most eukaryotes, kinesin-5 motors are essential for this process, and genetic or chemical inhibition of their activity leads to the emergence of monopolar spindles and cell death. However, these deficiencies can be rescued by simultaneous inactivation of kinesin-14 motors, as they counteract kinesin-5. We conducted detailed genetic analyses in fission yeast to understand the mechanisms driving spindle assembly in the absence of kinesin-5. Here we show that deletion of the nrp1 gene, which encodes a putative RNA-binding protein with unknown function, can rescue temperature sensitivity caused by cut7-22, a fission yeast kinesin-5 mutant. Interestingly, kinesin-14/Klp2 levels on the spindles in the cut7 mutants were significantly reduced by the nrp1 deletion, although the total levels of Klp2 and the stability of spindle microtubules remained unaffected. Moreover, RNA-binding motifs of Nrp1 are essential for its cytoplasmic localization and function. We have also found that a portion of Nrp1 is spatially and functionally sequestered by chaperone-based protein aggregates upon mild heat stress and limits cell division at high temperatures. We propose that Nrp1 might be involved in post-transcriptional regulation through its RNA-binding ability to promote the loading of Klp2 on the spindle microtubules.