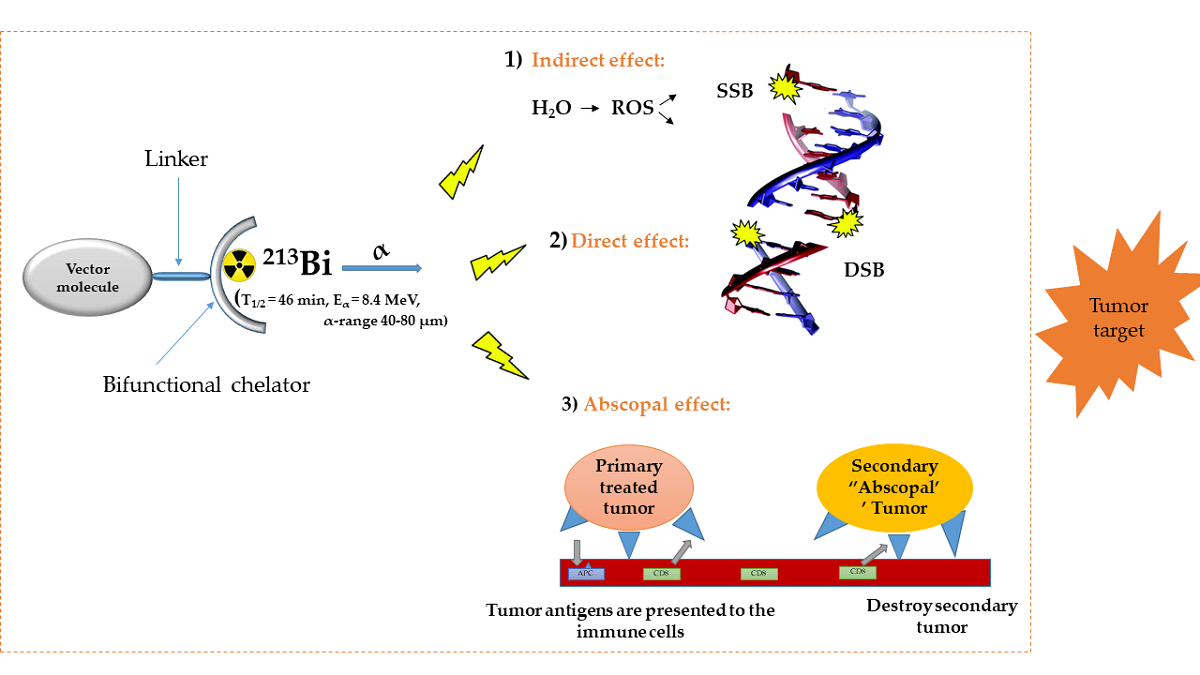
Besides external high-energy photon or proton beam therapy, targeted radionuclide therapy (TRNT) is an alternative approach to deliver radiation to cancer cells. TRNT is distributed within the body by the vascular system and allows targeted irradiation of a primary tumor and all its metastases, resulting in substantially less collateral damage to normal tissues as compared to ex-ternal beam radiotherapy (EBRT). It is a systemic cancer therapy, tackling systemic spread of the disease, which is the cause of death in most cancer patients. The α-emitting radionuclide bis-muth-213 (213Bi) has interesting properties and can be considered as a magic bullet for TRNT. The benefits and drawbacks of targeted alpha therapy with 213Bi are discussed in this review, covering the entire chain from radionuclide production to bedside. First, the radionuclide properties and production of 225Ac and its daughter 213Bi are discussed, followed by the fundamental chemical properties of bismuth. Next, an overview of available acyclic and macrocyclic bifunctional chelators for bismuth, and general considerations for designing a 213Bi-radiopharmaceutical are provided. Finally, we will provide an overview of preclinical and clinical studies involving 213Bi-radiopharmaceuticals, as well as the future perspectives of this promising cancer treatment option.