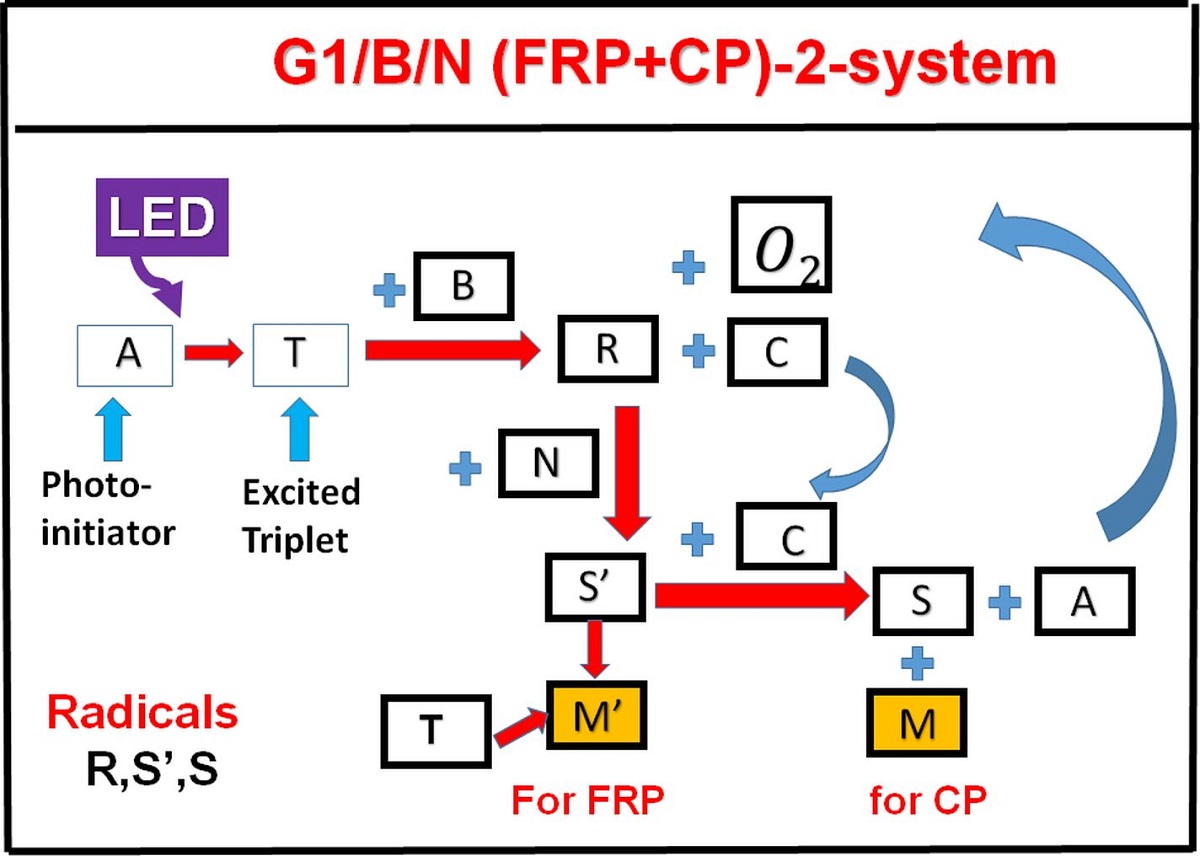
This article presents, for the first time, the kinetics and the general conversion features of a 3-initiator system (A/B/N), based on proposed mechanism of Mokbel et al, for both free radical polymerization (FRP) of acrylates and the free radical promoted cationic polymerization (CP) of epoxides using copper complex as the initiator. Higher FRP and CP conversion can be achieved by co-initiators concentration [B] and [N], via the dual function of (i) regeneration [A], and (ii) generation of extra radicals S' and S. The FRP and CP conversion is proportional to, respectively, the nonlinear and linear power of bI[A][B], where b and I are the absorption coefficient and the light intensity, respectively. System in air has lower conversion than in laminate due to the oxygen inhibition effects. For thick samples (with thickness z), there is an optimal concentration [A*] which is inverse proportional (bzI), in contrast with very thin sample, in which the conversion is an increasing function of [A] and [B]. The unique feature of dark polymerization in CP conversion enables the polymerization to continue in living mode, in contrasts with that of the radical-mediated pathway in most conventional FRP. The measured results of Mokbel et al are well analyzed and matching the predicted features of our modeling.