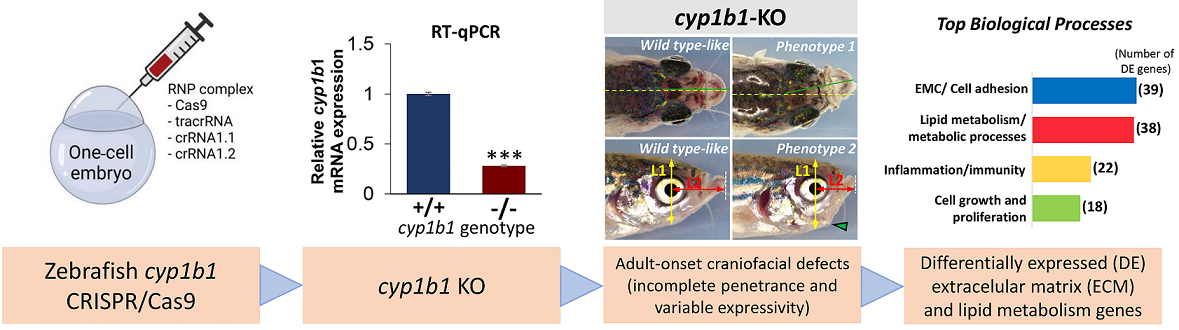
CYP1B1 loss-of-function (LoF) is the main known genetic alteration present in recessive primary congenital glaucoma (PCG), an infrequent disease characterized by delayed embryonic development of the ocular iridocorneal angle and caused by poorly understood molecular mechanisms. To model CYP1B1 LoF underlying PCG, we developed a cyp1b1 knockout (KO) zebrafish line using CRISPR/Cas9 genome editing. This line carries the c.535_667del frameshift mutation that results in a 72% mRNA reduction with residual mRNA predicted to produce an inactive truncated protein (p.(His179Glyfs*6)). Craniofacial defects and jaw maldevelopment were observed in 23% of somatic mosaic F0 crispant larvae (144 hpf). These early phenotypes were not detected in KO F3 larvae (144 hpf) but 27% of adult fishes (4 months) showed uni or bilateral craniofacial alterations, indicating the existence of incomplete penetrance and variable expressivity. These phenotypes increased to 86% in the adult offspring of inbred progenitors with craniofacial defects. No glaucoma-related phenotypes were observed in the cyp1b1 mutants. Transcriptomic analyses of the offspring (7dpf) of KO cyp1b1 progenitors with adult-onset craniofacial defects revealed that differentially expressed genes were functionally enriched in groups related with extracellular matrix and cell adhesion, cell growth and proliferation, lipid metabolism (retinoids, steroids, and fatty acids, and oxidation-reduction processes which included several cytochrome P450 genes) and inflammation. In summary, this study shows the complexity of phenotypes and molecular pathways associated with cyp1b1 LoF, with species-dependency, and provides evidence for dysregulation of extracellular matrix gene expression as one of the mechanisms underlaying pathogenicity associated with cyp1b1 disruption.