INTRODUCTION
In recent years, scientists have done a lot to find out the best way to produce through different experiments, and although they often succeeded, still some of the challenges have remained unsolved like the high consumption of energy in some reactions. Furthermore, sending equipment to other planets is not only harmful to Earth, but also it is so expensive that we get encouraged to find a chemical reaction that can be executed on another planet without a severe need for anything that comes from our home planet, Earth.
To go more in-depth, let’s study one of the NASA’s successful operations for producing on Mars, and analyze its benefits and disadvantages.
MOXIE
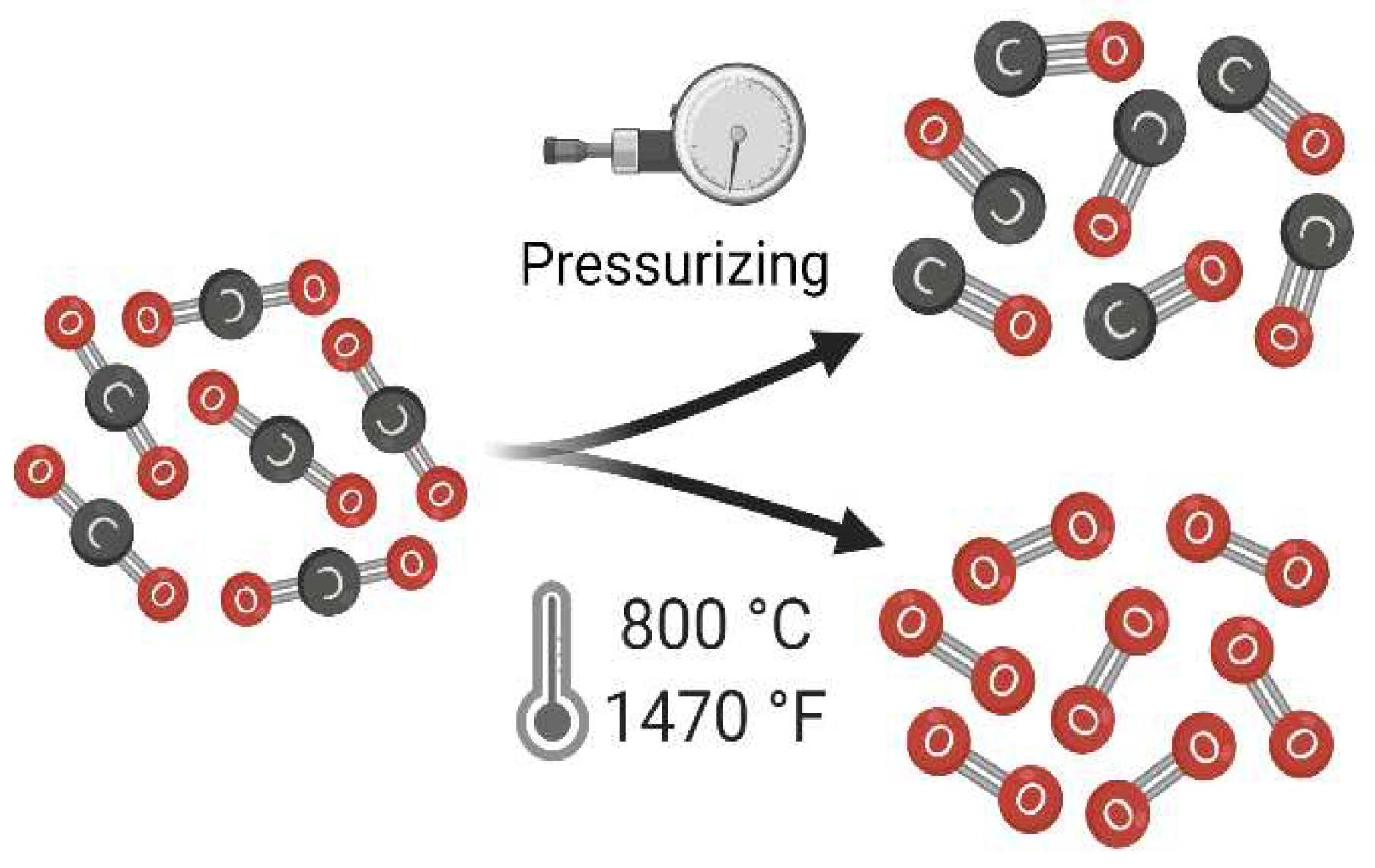
On February 18th, 2021, the Perseverance rover landed on Mars successfully, and it was also carrying a small helicopter that led humanity to fly on another planet for the first time! Anyhow, an important device called MOXIE was embedded inside the rover (front, right side). In summary, MOXIE stands for: “The Mars Oxygen In-Situ Resource Utilization Experiment”. A technology for investigating the production of oxygen on Mars based on the conditions of the Martian atmosphere. Carbon dioxide contains 95.32 percent of the Martian atmosphere and the remained amount consists of 2.7 percent nitrogen, 1.6 percent of argon, 0.13 percent of oxygen, and minor amounts of chemical mixtures like water, neon, krypton, xenon, and so on. Hence, MOXIE was exactly made to test the process of generating oxygen according to the following net reaction: . As a simple illustration, it can be viewed as
Figure 1.
A simple demonstration of how MOXIE performs the determined reaction.
Figure 1.
A simple demonstration of how MOXIE performs the determined reaction.
Although MOXIE produced grams of in the first attempt and that was a huge success, this amount of oxygen is not adequate to keep one person alive for more than a few minutes. It is true that MOXIE was just a prototype and it will be improved for sure, but the power consumption of this machine is which is equal to the all saved energy in the rover’s battery! So, what can be included here is that without considering other necessary facilities like heaters, pressure optimizers, lights, etc., we need to dedicate hundreds of square meters of solar panels to operate scaled-up versions of this technology.
Accordingly, let’s move forward and study the process of generating oxygen from water because experiments conducted in different space missions proved that water can be founded in some areas of the Moon and Mars!
DISCUSSION
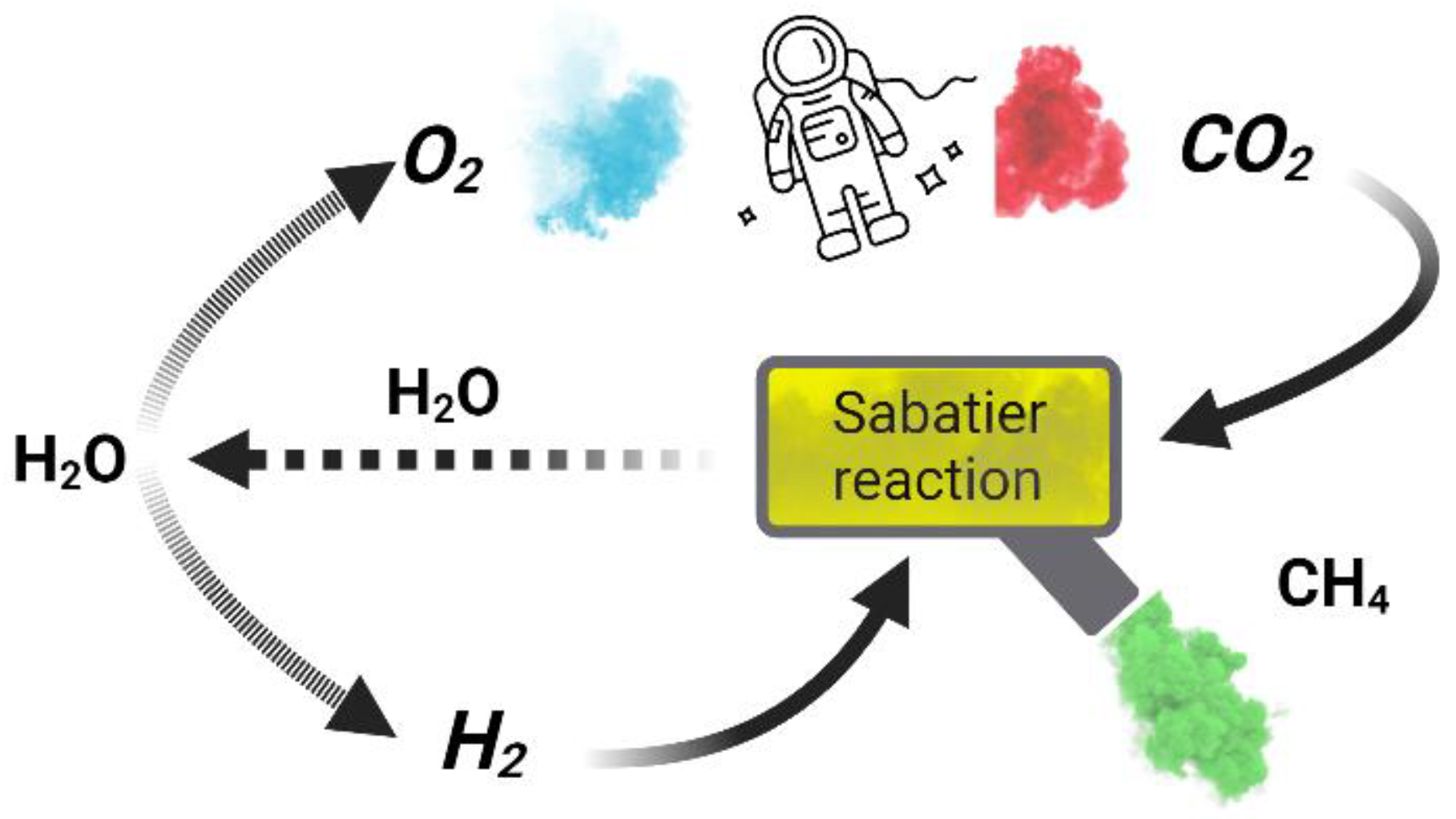
We need to design a system that at the beginning melts the ice obtained from underneath the surface of the Moon or Mars and then, split water into its components ( & ). Something unique to this approach is that we also gain , and it is a key to recycling the evolved from the human’s lungs, into breathable oxygen again! A comprehensive figure of these details can be shown as
Figure 2.
A holistic review of the proposed system.
Figure 2.
A holistic review of the proposed system.
In this system, the released methane () might not be used as a fuel because it consumes oxygen to a great extent. However, I believe that we can build an isolated storage made of glass and keep the obtained methane in this environment because methane is a powerful greenhouse gas, so with this, we can keep our storage warm in order to facilitate the process of melting ice. This improves the efficiency of our system as long as we no longer require to consume a high volume of energy to melt the ice that we extract from beneath the surface of the Moon and Mars.
METHODS
After melting ice, we consider the well-known water electrolysis method to split into , and which is a product that we have been focusing on from the beginning of this research. we require a theoretical minimum of electric energy to dissociate each mole of in the standard condition (STP) so with respect to the stochiometric rules, 1 mole of gives us 8 grams of !
Furthermore, we need a great catalyst like the Nickel [
2] Hydroxide, and the good news here is that this substance can be found on Mars in abundance! Additionally, for other environments such as Moon, we can refer to the information that researchers collected at the University of Houston.
They have found a promising oxygen-evolving catalyst with superior catalytic performance. It is highly active and stable, and it increases the speed and the efficiency of the reaction. In addition to that, by using this electrocatalyst, the amount of electric potential difference can be reduced and it is the best catalyst that can be used in the electrolysis of water on large scales. Refer to the references of this paper for gaining more information regarding this 3D foam of .
Anyway, now that we have produced oxygen and hydrogen, the astronauts’ lungs consume the breathable oxygen and spread out carbon dioxide. Here, we need to find a way to make the most out of the hydrogen that we gained in the primary stages, and the released carbon dioxide.
After reviewing some of the possible reactions, the Sabatier reaction attracts our attention! Sabatier reaction produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures of 300 - 400 °C optimally. In addition to that, it needs a pressure of perhaps 30 bar in the presence of a nickel catalyst. To have a better understanding, we can display the net reaction as
The above reaction was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens at the end of the 19th century. Let us point out that , as a product of this reaction, returns to the cycle and methane, as another product of this reaction, goes into the ice storage that we built out of glass in order to that melt the ice easier due to the greenhouse effect.
Something remarkable about the Sabatier reaction is that its enthalpy improves the efficiency of our Oxygen Generating System (OGS)! The reason is that whenever , the reaction will be done automatically and it naturally releases heat. The energy evolved from this reaction can get back to the stage where the water electrolysis is happening and therefore, it improves the efficiency of the whole cycle. As the author of this article, I also suggest the use of plants for recycling the extra amount of carbon dioxide as far as the Sabatier reaction is not 100% efficient.
Currently, the International Space Station recycles more than 90 percent of its water but is only able to recycle 40 percent of the total oxygen. As a result, these are the resupply missions that satisfy the astronauts’ needs for oxygen besides bringing food, water, and all essential materials for humanity’s survival. Nevertheless, this is a costly process specially in the cases that payloads have to travel a long distance to reach their destinations such as Mars or the Moon. To give an example. even carrying a bottle of water to the International Space Station which is in low earth orbit can cost something around $9,100 to $43,180!
Logically, damaging our home planet in exchange for colonizing other planets is never acceptable. So, a stable cycle with the highest efficiency is required and it is the exact reason why we designed a sustainable life cycle.
Finally, while the system that we proposed in this paper was just a prototype and may have a number of weaknesses besides their benefits, advancement of science can make the OGS much more efficient, and this will eventually make it excellent enough to be installed in future space colonies.
Acknowledgements
I am really grateful to all of those who taught me to learn and I was honored to meet them.
References
- Eric Hinterman. “Simulating Oxygen Production on Mars for MOXIE (Mars Oxygen In-Situ Resource Utilization Experiment)”, ResearchGate, 2018. [CrossRef]
- 2. Kaitlin Sullivan. “How the ISS recycles its air and water”, Popular Science, 2019, URL: https://www.popsci.com/how-iss-recycles-air-and-water/.
- Haiqing Zhou, Fang Yu, Jingying Sun, Ran He, Shuo Chen, Ching-Wu Chu, Zhifeng Ren, “Fe (PO3)2/Ni2P for efficient water oxidation”, Proceedings of the National Academy of Sciences, May 2017.
- Takashi Yoshizaki & William F McDonough, “The composition of Mars”, ResearchGate, January 2020, URL: https://www.researchgate.net/publication/335880205_The_composition_of_Mars.
- Sharp R. (1974). “Ice on Mars” Journal of Glaciology, Cambridge university press, January 2017, 13(68), 173-185. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).