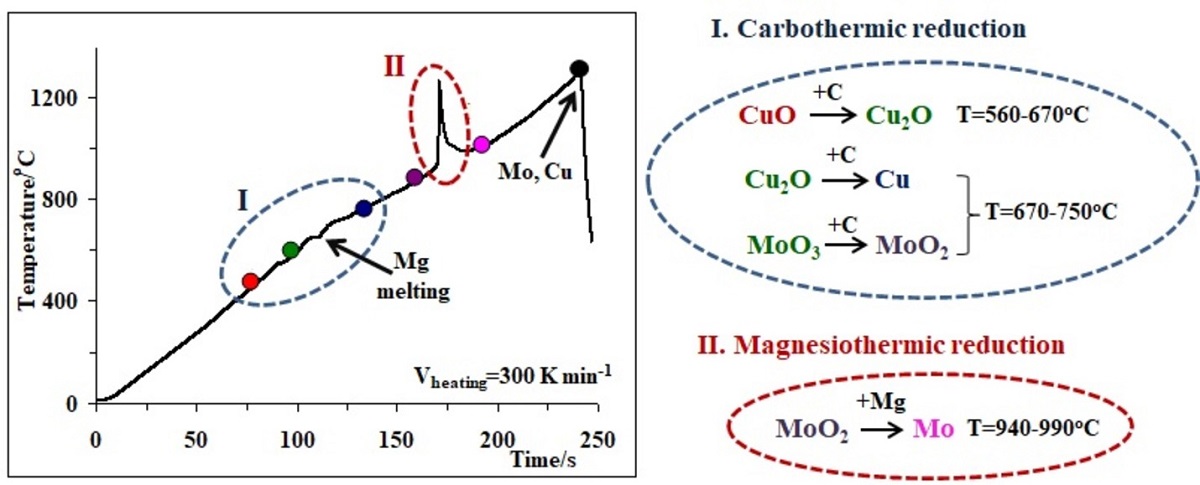
Understanding of the decisive role of the interaction mechanism and kinetics in the combustion processes is highly relevant for the elaboration of optimal conditions for obtaining Mo-Cu composite powders. From this perspective, the efficient delivery of the reduction mechanism of copper and molybdenum oxides with combined Mg + C reducing agents at high heating rates is crucial to develop a valuable approach for the combustion synthesis of Mo-Cu composite powders. Herein, we shed light on the mechanism of the reactions in all the studied binary, ternary and quaternary systems contemporaneously demonstrating the effect of the heating rate on the conversion degree. The combination of two highly exothermic and speedy reactions (MoO3+3Mg and CuO+Mg vs MoO3+CuO+4Mg) led to a slow interaction with weak self-heating (dysynergistic effect) due to a change in the reaction mechanism. On the other hand, it has been shown that during the simultaneous utilization of the Mg and C reducing agents, the process begins exclusively with carbothermic reduction, and at relatively high temperatures it continues with magnesiothermic one. The effective activation energy values of the magnesiothermic stages of the studied reactions were determined by Kissinger isoconversional method.