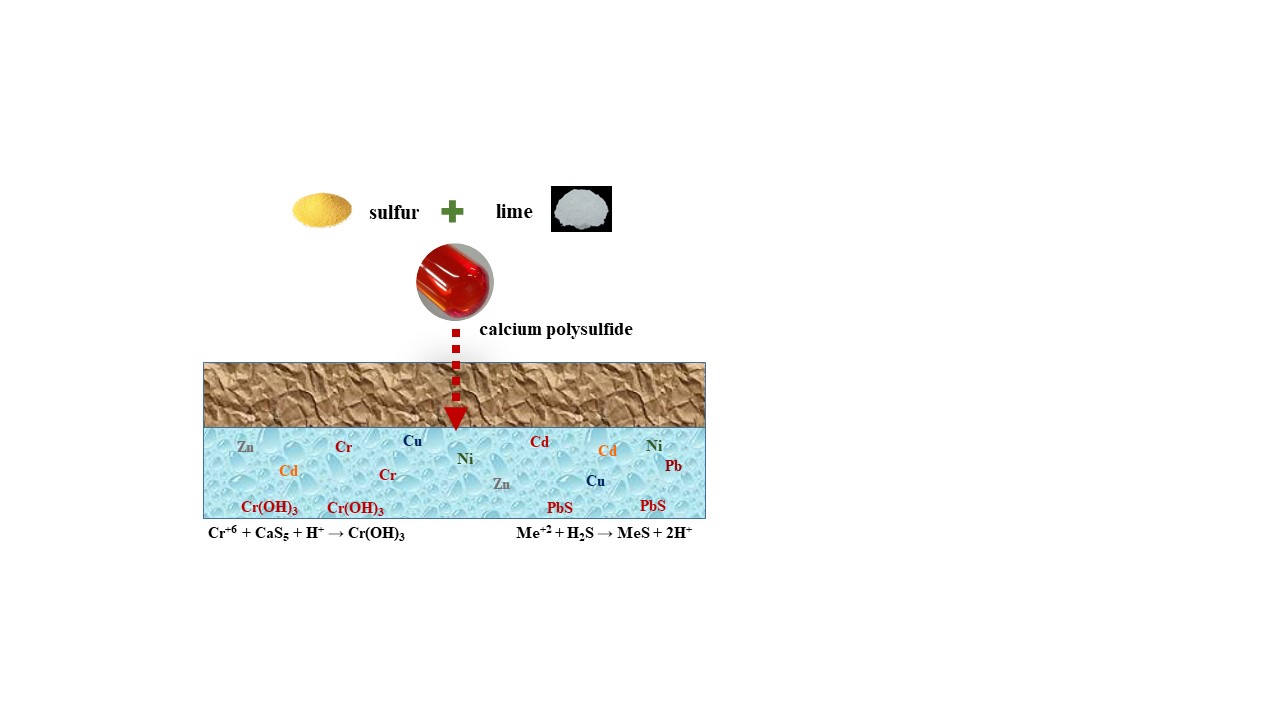
Heavy metals contamination in groundwater often occurs in various industrial processes. Stud-ies have confirmed that polysulfide could reduce hexavalent chromium to trivalent chromium, achieving the effect of in-situ stabilization. For other heavy metals contamination in groundwa-ter, whether polysulfide also had a stabilizing ability to achieve in-situ remediation. This re-search focused on heavy metals except for chromium that often contaminated in groundwater, including lead, nickel, zinc, copper, and cadmium to explore the feasibility of using calcium polysulfide (CaSx) as an in-situ stabilization technology for these heavy metals contamination groundwater. Results showed that CaSx had a great removal efficiency for heavy metals lead, nickel, zinc, copper, and cadmium. However, for nickel, zinc, copper and cadmium, when CaSx was added excessively, complexes would be formed, causing the result of re-dissolve and this would also reduce the removal efficiency. Since it is difficult to accurately control the dosage of agents for in-situ groundwater remediation, the concentration of re-dissolved nickel, zinc, cop-per, and cadmium may not be able to meet the groundwater control standards. CaSx had high lead removal efficiency, and it would not cause re-dissolution due to excessive CaSx dosing. CaSx can be used as an in-situ stabilization technique for lead contaminated groundwater.