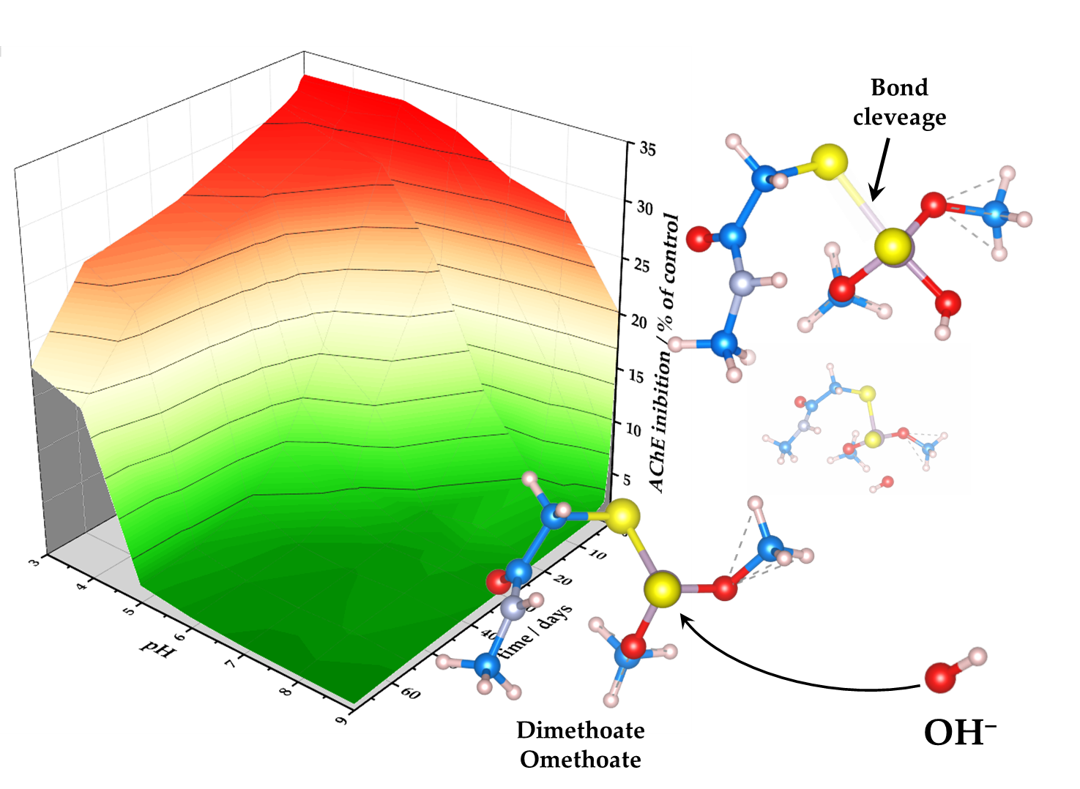
Organophosphate pesticides are used in large quantities. However, they exhibit toxic effects on non-target organisms. Dimethoate and its oxo-analog omethoate inhibit acetylcholinesterase and are toxic for mammals. Moreover, they show extreme toxicity for bees. Once in the environment, they undergo chemical transformations and decomposition. We show that dime-thoate and omethoate decompose rapidly in alkaline aqueous solutions (half-lives 5.7 and 0.89 days) but are highly stable in acidic solutions (half-lives 124 and 104 days). These differences are explained using quantum chemical calculations, indicating that a weaker P–S bond in omethoate is more susceptible to hydrolysis, particularly at a high pH. The toxicity of these pesticides solutions decreases over time, indicating that no or very little highly toxic omethoate is formed during hydrolysis. Presented data can be used to predict dimethoate and omethoate concentrations in contaminated water depending on pH. Presented results suggest that alkaline hydrolysis of organophosphates has an advantage over other techniques for their removal since there is no risk of omethoate accumulation and increased toxicity of contaminated water.