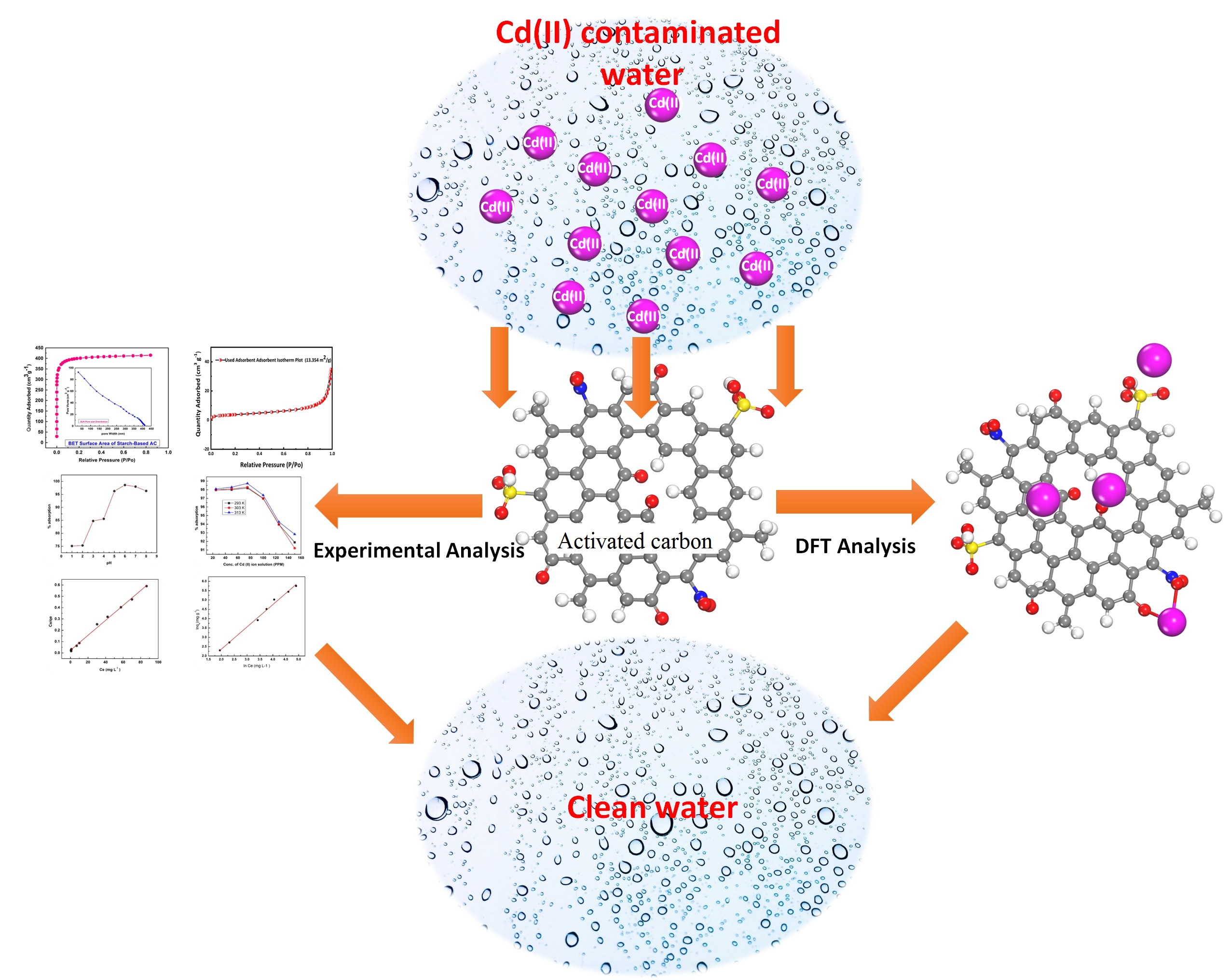
Cadmium(II) contamination in the environment is an emerging problem due to its acute toxicity and mobility, so it is very urgent to remove this species from industrial wastewater before it is discharged into the environment. Thus, a starch-based activated carbon (AC) with a specific surface area of 1600 m2g-1 is used as an adsorbent for the capturing of toxic cadmium(II) ions from synthetic solutions. The sorbent is characterized by BET, SEM, TEM, XRD, FT-IR, TGA, and zeta potential. The maximum uptake (284 mg g-1) of Cadmium(II) ion is obtained at pH 6. The thermodynamic parameters like ∆G, ∆H, ΔS are found to be -17.42 kJmol-1, 6.49 kJ mol-1, and 55.66 Jmol-1K-1 respectively, revealing that the adsorption mechanism is endothermic, spontaneous, and feasible. The experimental data follows the D-R and Langmuir models well. The mass transfer is controlled by pseudo 2nd order kinetics. Furthermore, the density functional theory simulations demonstrate that the activated carbon strongly interacted with the Cd(II) ion through its various active sites. The adsorption energy noted for all interactive sites is highly negative (-0.45 eV to -10.03 eV), which shows that the adsorption process is spontaneous and stable which is in agreement with the experimental thermodynamics analysis.