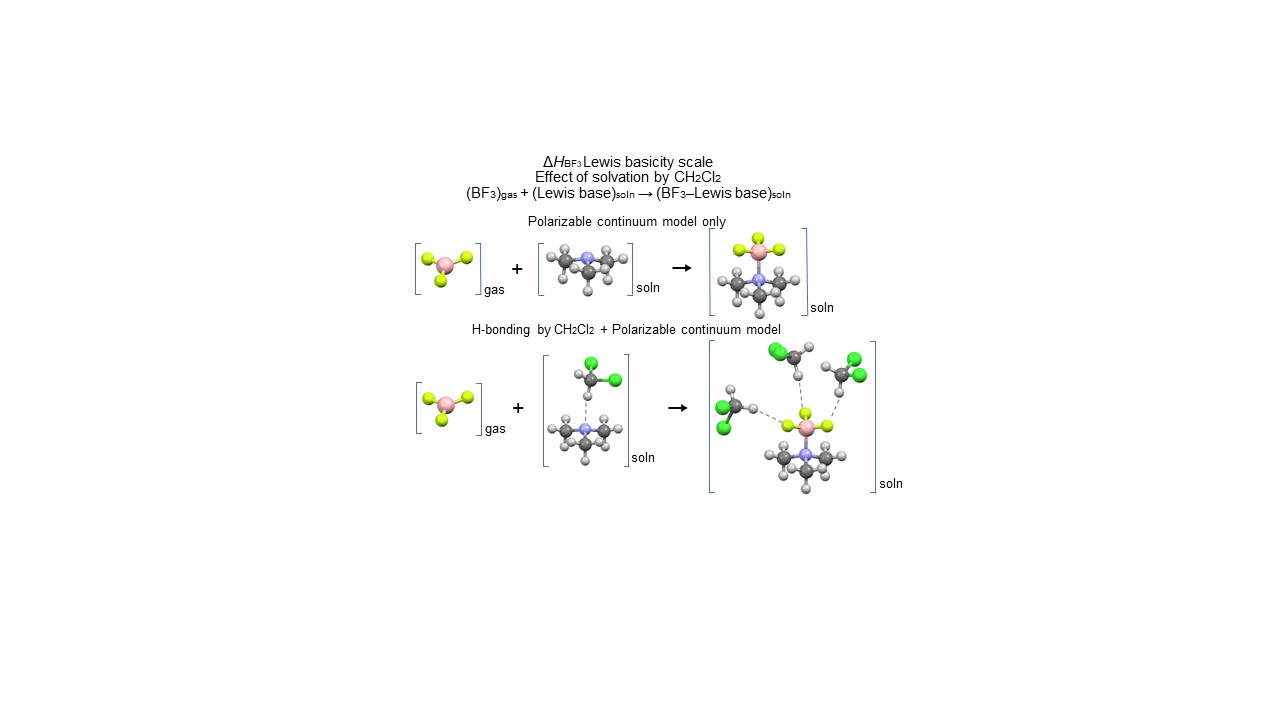
The Lewis basicity of selected organic bases, modeled by the enthalpies of adduct formation between gaseous BF3 and the bases in dichloromethane (DCM) solution, is critically examined. Although experimental enthalpies for a large number of molecules have been reported in the literature, it may be desirable to estimate missing or uncertain data for important Lewis bases. We have decided to use high-level ab initio procedures, combined with a polarized continuum solvation model, in which the solvated species are the clusters formed by specific hydrogen bonding of DCM with the Lewis base and the Lewis base/BF3 adduct. This mode of interaction with DCM corresponds to a specific solvation model (SSM). The results actually show that the enthalpy of BF3 adduct formation in DCM solution is clearly influenced by specific interactions, DCM acting as hydrogen-bonding donor (HBD) molecule in two ways: base/DCM and adduct/DCM, confirming that specific solvation is an important contribution to experimentally determined Lewis basicity scales. This analysis allows us to conclude that there are reasons to suspect some gas-phase values to be in error by more than the stated experimental uncertainty. Some experimental values in DCM solution that were uncertain because of identified reasons can be complemented by the computed values.