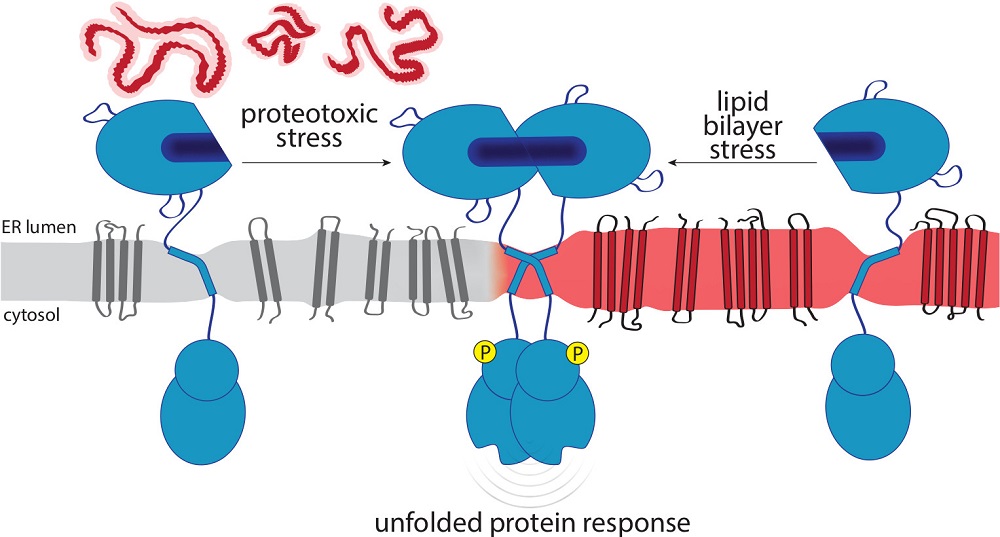
The endoplasmic reticulum (ER) is the major site of membrane biogenesis in most eukaryotic cells. As the entry point to the secretory pathway, it handles more than 10.000 different secretory and membrane proteins. The membrane insertion of proteins, their folding, and ER exit are affected by the lipid composition of the ER membrane and its collective membrane stiffness. The ER is also a hotspot of lipid metabolism for membrane lipids including sterols, glycerophospholipids, ceramides and neural storage lipids. The unfolded protein response (UPR) bears an evolutionary conserved, dual sensitivity to both protein folding-imbalances in the ER lumen and aberrant compositions of the ER membrane, referred to as lipid bilayer stress (LBS). Through transcriptional and non-transcriptional mechanisms, the UPR upregulates the protein folding capacity of the ER and balances the production of proteins and lipids to maintain a functional secretory pathway. In this review, we discuss how UPR transducers sense unfolded proteins and LBS with a particular focus on their role as guardians of the secretory pathway.