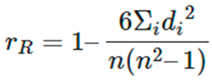Introduction
Hepatitis C virus (HCV) it is know as a hepatic pathology in extension nowadays, accounts for approximately 15%-20% cases of acute hepatitis.[
1,
2,
3,
4]The aim of clinic diagnostic of viral infection in human polulation, is to find the infected persons and to treat. Virological methods are used practically foe a proper diagnostic. [
5] Currently the third generation test of EIA for the anti-HCV antibody detection is commonly used for laboratory diagnostic.[
6] For this purpose, EIAs are easy to use. More than, we can mention that EIAs and anti-HCV antibody are rutinally recommended for screening in HCV infections to different groups of polulation.[
7] Good to mention that HCV subtyping play a great role for epidemiological studies.[
8]
The method for HCV genotyping is genome sequencing. In this context we can mention the core/E1 or the NS5B regions and subsequent analysis.[
9] From a research perspective a point in HCV infection diagnosis is based on the detection of antibodies to recombinant HCV polypeptides and by assays for HCV- RNA. Practically, enzyme immunoassays play a great rotinally role and within rheir, is possible to measure antibodies. In attention of previusly methods are NS4, core, NS3, and NS5 sequences. Priori establishing the HCV treatment, the next step is to stage the disease. For this purpose practically is utilizing liver biopsy, concretly gold standard method.In adition, imaging modalities with or without noninvasive biomarkers also are practically important.[
10,
11,
12] In HCV infection, a proper treatment is important in order to treat specifically each ill person. [
13,
14] From another perspective, oxidative stress is reported as an important part of HCV which produce liver alterations.[
15]The presence of oxidative stress has been identify in types of hepatitis that include also hepatitis B [
16] From results of various studies, we can mention that HCV-core and non-structural components, NS3 and NS5A proteins, directly induce OS. [
17,
18,
19] A specific group of research studies, show us that HCV-induced steatosis is also in attention.nowadays. [
20] For sure, actually it is know that hepatitis C Virus (HCV) is a cause which conduct to alterations in the liver normally structure and then concure to the chronic liver disease. [
21] So, following negative steps, chronic infections with HCV five us result that are show us alterations in the normally liver structure, as inflammation in the liver tissue. The proper treatment practically used in HCV infection, it is know because reduce the long-term complications, cirrhosis, HCC and causes of mortality. [
22,
23,
24,
25,
26,
27,
28,
29] Nowadays, from various liver diseases, hepatitis C it is know as a global health problem as the World Health Organization (WHO) mention and establish. In this direction, in different developing countries, public health officials do not understand the true burden of infection with hepatitis C Virus.[
30,
31]
Figure 1.
Liver H&E staining x10.
Figure 1.
Liver H&E staining x10.
Figure 2.
Liver H&E staining x10.
Figure 2.
Liver H&E staining x10.
Material and Methods
For this study, investigations were in the Olymp Clinical Diagnostic Laboratory, Karaganda, from October 2016 to December 2016. For the purpose of the study, 6000 automatic modular analyzer ( Roche Diagnostics model), were used for ECL analysis.
For screening patients with a high risk of hepatitis C, we conducted two effective and possible in our conditions tests of ECL and ELISA of the third generation. ECL is a process of electrogenerated chemiluminescence that combines the advantages of both electrochemical and photoluminescence analysis.The ECL method possesses excellent characteristics such as, speed of response, economic, simple operation processes and high sensitivity, and has been widely used in the detection of antibodies.In contrast, ELISA is more sensitive and specific than Chemiluminescence for blood transfusion screening. Third-generation ELISA screening is recommended for confirmatory tests for HCV antibodies.
A Bio RAD immunoassay analyzer, were used for ELISA method. (Enzyme-Linked Immunosorbent Assay). In this laboratory investigation, there are using a set of reagents Vector-BEST (Russia) for detecting total antibodies to each of the 4 antigens of the HCV, concretly the core (core) and non-structural proteins (NS3, NS4, NS5). Interpretation for conformation: tests with a positive result for the core antigen, or with two or three non-structural proteins (NS3, NS4 or NS5) were considered as positive.
Enzyme-Linked Immunosorbent Assay technique .results, were evaluated by optical density (OD) using a microplate spectrophotometer (BioRAD). Critical optical density (COD) is equal to the half-sum of optical density values of two negative control samples plus a correction factor.
Procedure Steps:
Pipetting of the sample, reagent and microparticles.
The reaction mixture is supplied for measurement.
Each cycle is performed within 42 seconds.
The number of pipetting steps and preparation of the reaction mixture depend on the test.
Some tests require dilution with a diluent, which increases the number of pipetting steps. The incubation time at 37 °C ranges from 4.5 to 9 minutes, depending on the test.
Calibration and quality control in ECL. Calculation of the calibration curve was carried out at the time of production of the reagent and it wasencoded in a 2-dimensional bar code of the corresponding set with the reagent. This information is then read by the analyzer.Monitoring the operation of the analyzer was conducted with 2 levels of controls, normal and pathological. Calibration and quality control were performed.
Results for the ECL assay were expressed as a signal to cut-off (s/co) ratio; a s/co ratio<0.9 was a negative and s/co ratio ≥1.0 indicated positive result. A s/co ratio within 0.9 and <1.0 was considered to fall into “a gray zone” and these serum samples were considered negative for calculations this study.
Interpretation for conformation: tests with a positive result for the core antigen, or with two or three non-structural proteins NS3, NS4 or NS5 were considered as positive.
Results:
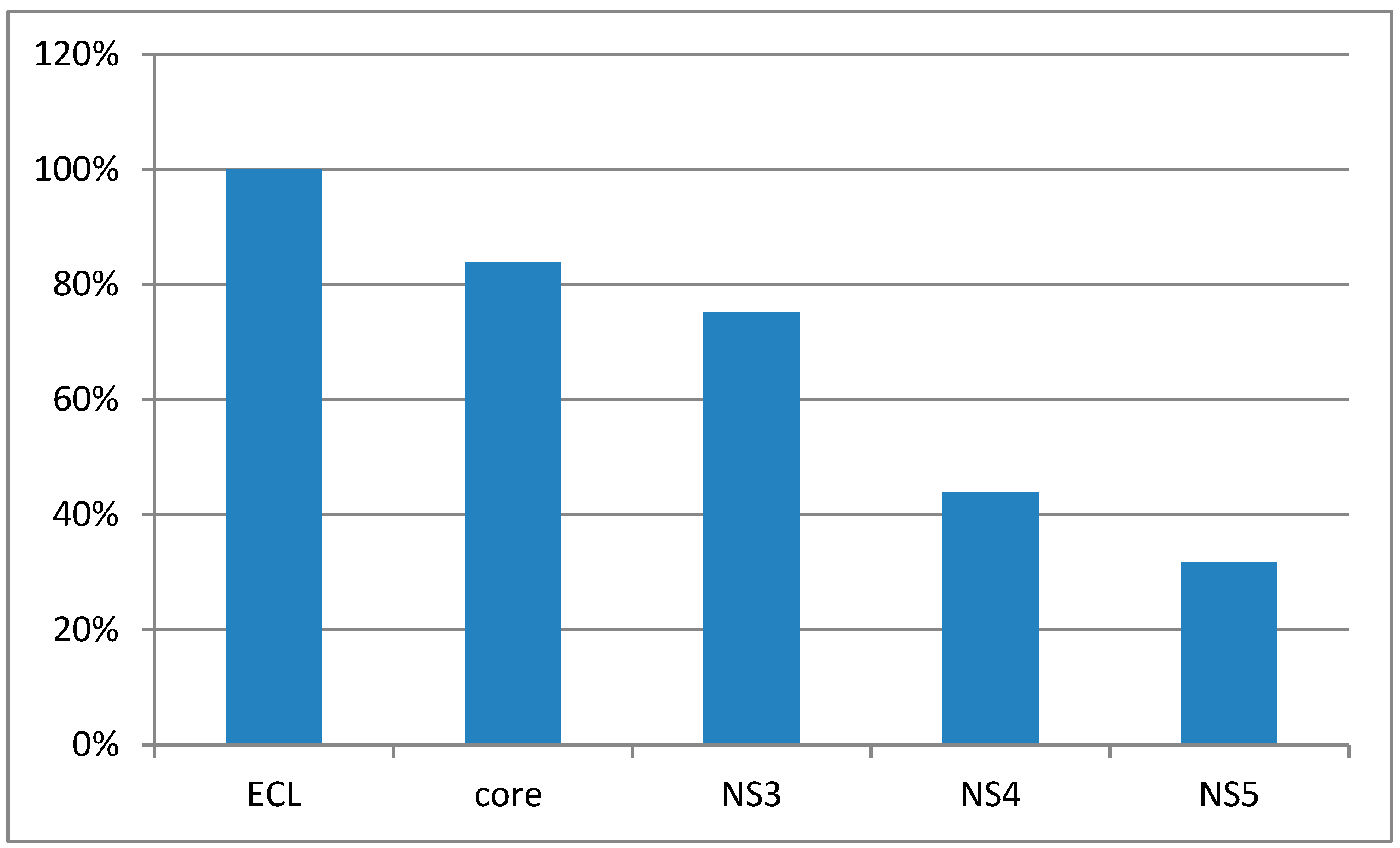
Knowing the data collated in 2016, we can conclude after results of the study, that the incidence of the HVC, is higher than in the one of the previous period studied, respectively, 2004-2009. Electrochemiluminescence method and Enzyme-Linked Immunosorbent Assay method, are not able to show unclear results as false negative or false positive, in HCV diagnostic.The detection rate of antibodies to non-structural antigens NS3, NS4, and NS5 ranged from lower to higher.The positive results of antibodies to NS3 were significantly higher compared to NS4 and NS5 At the same time NS4 and NS5 did not differ significantly. [Figure 1]
Figure 1.
The percentage of positive samples in the EСL test and ELISA depending on antibody markers.
Figure 1.
The percentage of positive samples in the EСL test and ELISA depending on antibody markers.
As presented in Figure 1, of the 205 samples with positive ECLtest, antibodies to core-antigen were detected in 172 samples.
The diagram shows that the incidence rate of hepatitis C in both Karaganda and the Republic standshigh (at 12.22 and 9.12 respectively) in 2016 compared with the period 2004-2009.
Table 2.
Values of S / CO ratio in positive samples.
Table 2.
Values of S / CO ratio in positive samples.
| S/CO ratio |
1-4 |
5-10 |
11-20 |
21-30 |
31-40 |
41-50 |
51-60 |
61-100 |
100 > |
Total |
| n |
17 |
19 |
35 |
48 |
29 |
22 |
16 |
15 |
4 |
205 |
| % |
8,29 |
9,27 |
17,07 |
23,42 |
14,15 |
10,73 |
7,81 |
7,31 |
1,95 |
100 |
We noted the nonparametric distribution of the values of S / COat Me30,02 (Q75-Q25 49,14-16,89).205 positive blood samples were sent for confirmation in the ELISA test.
Table 3.
The significance of differences of the antibodies to non-structural antigens in ELISA in patients at risk for HCV.
Table 3.
The significance of differences of the antibodies to non-structural antigens in ELISA in patients at risk for HCV.
| A-body |
n |
p% |
m% |
Significance of differences |
| NS3 and NS4 |
NS4 and NS5 |
NS3 and NS5 |
| z1-2 |
p-level |
z2-3 |
p-level |
z1-3 |
p-level |
| NS3 |
154 |
75,12 |
3,48 |
4,97 |
0,0000* |
1,57 |
0,119 |
6,44 |
0,0000* |
| NS4 |
90 |
43,9 |
5,23 |
|
|
|
|
|
|
| NS5 |
65 |
31,7 |
5,77 |
|
|
|
|
|
|
As a specific table nr. 3, show us that the correlations antibodies detected in ECL and ELISA are outlined. More than, previously mentioned correlations were used to identify any possible relationships between ECL index and indicators of ELISA, as set out in another specific below table. The correlations between the ECL ratio and core antibody were moderate (r=0,54), and no relationships between ECL ratio antibodies to nonstructural NS3, NS4, NS5 antigens. The correlations between core antibody and NS3, NS4, NS5 antibodies varied from moderate to weak (r=0,48; 0,42;0,32) respectively.The largest correlations is between NS3 and NS4 (r=0,67) , also between NS5 and NS3, NS4 (r= 0,63).
It is important to test vein blood using RT–PCR technique (Reverse transcription polymerase chain reaction), in order to exclude false results. Antibodies detected in blood samples and HCV core antigen play a signifiant role in a diagnostic for HCV infection[
16]. In this context antibodies to NS3 are specific for diagnosis the early stages of hepatitis C. Also NS3 is considered as an independent diagnostic marker of the HCV acute process. The number of positive samples with NS4 and NS5 antibodies significantly less in comparison with NS3, show us in this study that a higher number of patients were in the acute form of HCV. The cronicisation process of the liver distruction could be relevant studing in the laboratory, exactly, anti-NS4 and anti-NS5.The study show also differents correlative results between the ECL ratio and antigens as NS3, NS4, NS5.
Statistical Analysis was performed using the laboratory information system LIS (Moscow, Russia) and the online calculator medstatistic.ru was used to calculate median (Me), lower (Q25) and upper (Q75) quartiles. A non-parametric Spearman correlation coefficient was used to determine data correlation:
Spearman correlation coefficient: Formula and Calculation with Example.
Table 1.
Statisical basis for Spearman correlation coefficient (Adapted from Dencey and Reidy 2011).
Table 1.
Statisical basis for Spearman correlation coefficient (Adapted from Dencey and Reidy 2011).
| Sperman (r) |
Correlation |
≥ 0,70
4-6
1-3 |
Strong relationship
Moderate relationship
Weak relationship |
Discussions
Knowing the data collated in 2016, we can conclude after results of the study, that the incidence of the HVC, is higher than in the one of the previous period studied, respectively, 2004-2009. Electrochemiluminescence method and Enzyme-Linked Immunosorbent Assay method, are not able to show unclear results as false negative or false positive, in HCV diagnostic. It is important to test vein blood using RT–PCR technique (Reverse transcription polymerase chain reaction), in order to exclude false results. Antibodies detected in blood samples and HCV core antigen play a signifiant role in a diagnostic for HCV infection[
16].
In this context antibodies to NS3 are specific for diagnosis the early stages of hepatitis C.
Also NS3 is considered as an independent diagnostic marker of the HCV acute process. The number of positive samples with NS4 and NS5 antibodies significantly less in comparison with NS3, show us in this study that a higher number of patients were in the acute form of HCV. The cronicisation process of the liver distruction could be relevant studing in the laboratory, exactly, anti-NS4 and anti-NS5.The study show also differents correlative results between the ECL ratio and antigens as NS3, NS4, NS5.
The increase in the incidence of HCVin KZ is associated with two trends: a real increase in the incidence and the consequence ofimprovements in diagnosis.According to the order of the Minister of Health of Kazakhstani N 451 of June 3, 2017viral hepatitis is included in the list of socially significant diseases that are dangerous to others.It should be noted that in recent years in RK, the detection of viral hepatitis has been associated with the expansion of the list of persons to be examined (medical workers, people before surgery, patients of centers and departments of hemodialysis, hematology, oncology, transplantation, cardiovascular and pulmonary surgery,drug users and others). Antiviral therapy of viral hepatitis is reflected in the order of the Minister of Health of the Republic of Kazakhstan dated May 4, 2019. We expect new prospects for reducing the incidence of viral hepatitis in the Republic of Kazakhstan, especially in the context of accurate and sensitive diagnosis of HCV infection being important.
Conclusions
The study showed that EСL and third generation of ELISA are important for screening and for conformation HCV risk patients.
Antibodies defined in the ECL test correlated with core antibodies ELISA are also good to know for a proper diagnostic.
The highest correlation were among antibodies to non-structural antigens. NS3, NS4, NS5 antibodies had an independent value for the differentiation of an acute or chronic process.
The study cannot conclude about false negative and false positive in this study. So unclear to find the presumptive enlarged “gray zone”, including false results.
RT-PCR technique is consider one of the modern and with high potential for the diagnosis of hepatitis C patients, in order to confirm or to exclude this disease.
WHO and national health organizations have a great project. WHO project purpose, is to eradicate HCV infection in the next coming 10 years. The global elimination of HCV before 2030 can be held if global and national health organizations build proper strategies.
Author Contributions
Conceptualization, A.M. and G.A.; methodology, G.A.; software, B.K.; validation, S.A.; formal analysis, A.C.; investigation, A.M; resources, A.M.; data curation, G.A.; writing—original draft preparation, A.C., T.S; writing—review and editing, A.C. T.S; visualization, G.A.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest.
References
- Carrozzo, M.; Scally, K. Oral manifestations of hepatitis C virus infection. World J Gastroenterol. 2014, 20, 7534–7543. [Google Scholar] [CrossRef]
- Ozkok, A.; Yildiz, A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014, 20, 7544–7554. [Google Scholar] [CrossRef]
- Grimbert, S.; Valensi, P.; Lévy-Marchal, C.; Perret, G.; Richardet, J.P.; Raffoux, C.; Trinchet, J.C.; Beaugrand, M. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case-control study. Gastroenterol Clin Biol. 1996, 20, 544–548. [Google Scholar]
- Montenegro, L.; De Michina, A.; Misciagna, G.; Guerra, V.; Di Leo, A. Virus C hepatitis and type 2 diabetes: a cohort study in southern Italy. Am J Gastroenterol. 2013, 108, 1108–1111. [Google Scholar] [CrossRef]
- Chevaliez, S.; Pawlotsky, J.M. How to use virological tools for optimal management of chronic hepatitis C. Liver Int. 2009, 29 (Suppl. S1), 9–14. [Google Scholar] [CrossRef]
- At present, the third generation test of EIA for the anti-HCV antibody detection is commonly used in the diagnostic laboratory.
- Campos-Outcalt, D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012, 61, 744–746. [Google Scholar]
- Schneider, M.D.; Sarrazin, C. Antiviral therapy of hepatitis C in 2014: do we need resistance testing? Antiviral Res. 2014, 105, 64–71. [Google Scholar] [CrossRef]
- Murphy, D.G.; Willems, B.; Deschênes, M.; Hilzenrat, N.; Mousseau, R.; Sabbah, S. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5’ untranslated region sequences. J Clin Microbiol. 2007, 45, 1102–1112. [Google Scholar] [CrossRef]
- Maticic, M.; Zorman, J.V.; Gregorcic, S.; Schatz, E.; Lazarus, J.V. Changes to the national strategies, plans and guidelines for the treatment of hepatitis C in people who inject drugs between 2013 and 2016: a cross-sectional survey of 34 European countries. Harm Reduct J. 2019, 16, 32. [Google Scholar] [CrossRef]
- Lam, J.O.; Hurley, L.B.; Chamberland, S.; Champsi, J.H.; Gittleman, L.C.; Korn, D.G.; Lai, J.B.; Quesenberry, C.P.; Ready, J.; Saxena, V.; Seo, S.I.; Witt, D.J.; Silverberg, M.J.; Marcus, J.L. Hepatitis C treatment uptake and response among human immunodeficiency virus/hepatitis C virus-coinfected patients in a large integrated healthcare system. Int J STD AIDS. 2019, 30, 689–695. [Google Scholar] [CrossRef]
- Oraby, M.; Khorshed, A.; Abdul-Rahman, E.; Ali, R.; Elsutohy, M.M. A clinical study for the evaluation of pharmacokinetic interaction between daclatasvir and fluoxetine. J Pharm Biomed Anal. 2019, 171, 104–110. [Google Scholar] [CrossRef]
- Mukhtar, N.A.; Ness, E.M.; Jhaveri, M.; Fix, O.K.; Hart, M.; Dale, C.; Pratt, C.; Kowdley, K.V. Epidemiologic features of a large hepatitis C cohort evaluated in a major health system in the western United States. Ann Hepatol. 2019, 18, 360–365. [Google Scholar] [CrossRef]
- Cunningham, H.E.; Shea, T.C.; Grgic, T.; Lachiewicz, A.M. Successful treatment of hepatitis C virus infection with direct-acting antivirals during hematopoietic cell transplant. Transpl Infect Dis. 2019, 21, e13091. [Google Scholar] [CrossRef]
- Clément, S.; Pascarella, S.; Negro, F. Hepatitis C virus infection: molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses. 2009, 1, 126–143. [Google Scholar] [CrossRef]
- Fujita, N.; Sugimoto, R.; Ma, N.; Tanaka, H.; Iwasa, M.; Kobayashi, Y.; Kawanishi, S.; Watanabe, S.; Kaito, M.; Takei, Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008, 15, 498–507. [Google Scholar] [CrossRef]
- Okuda, M.; Li, K.; Beard, M.R.; Showalter, L.A.; Scholle, F.; Lemon, S.M.; Weinman, S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002, 122, 366–375. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Ahmad, I.M.; Spitz, D.R.; Schmidt, W.N.; Britigan, B.E. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005, 76, 489–497. [Google Scholar] [CrossRef]
- Dionisio, N.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Majano, P.L.; Benedicto, I.; Rosado, J.A.; Salido, G.M.; Gonzalez-Gallego, J. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009, 50, 872–882. [Google Scholar] [CrossRef]
- Cheng, Y.; Dharancy, S.; Malapel, M.; Desreumaux, P. Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol. 2005, 11, 7591–7596. [Google Scholar] [CrossRef]
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- arrat F, Fontaine H, Dorival C, French ANRS CO22 Hepather cohort, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Van der Meer, A.J.; Veldt, B.J.; Feld, J.J.; et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J Am Med Assoc. 2012, 308, 2584–2593. [Google Scholar] [CrossRef]
- Nahon, P.; Bourcier, V.; Layese, R.; et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017, 152, 142–156. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017, 153, 996–1005. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Beste, L.A.; et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018, 69, 1088–1098. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018, 155, 411–421. [Google Scholar] [CrossRef]
- Li, D.K.; Ren, Y.; Fierer, D.S.; et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: an ERCHIVES study. Hepatology. 2018, 67, 2244–2253. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; et al. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2020, 71, 44–55. [Google Scholar] [CrossRef]
- Arab Republic of Egypt, Ministry of Health and Population National Committee for the Control of Viral Hepatitis. Egyptian national control strategy for viral hepatitis 2008-2012. [Accessed 2012 Jan 5] Available from: http://www.pasteur-international.org/ip/resource/filecenter/document/01s-000042-0da/nsp-10-april-2008-final.pdf.
- Ministry of Health, Government of Pakistan. Prime Minister’s Program for Hepatitis Prevention and Control phase I (2005-2010) and phase II (2010-2015) Report: 2010.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).