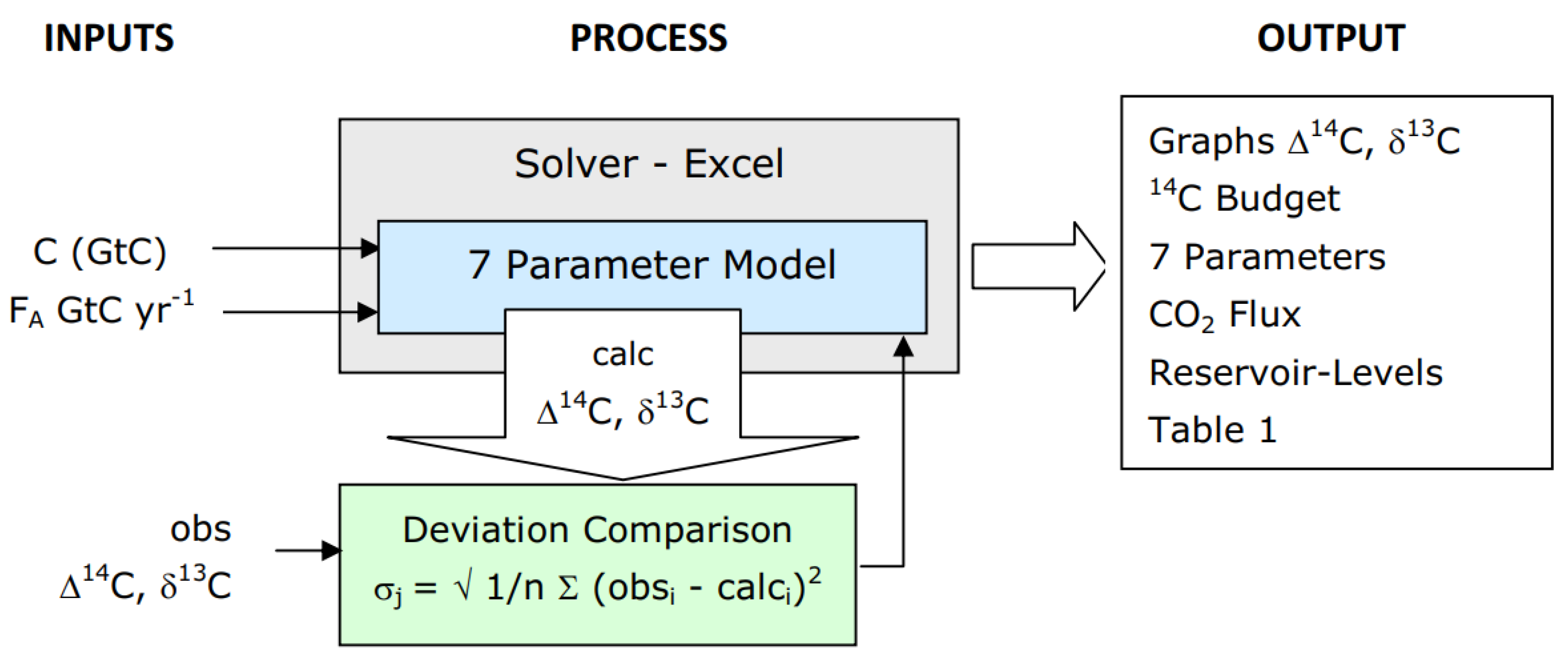
1. Introduction
The absolute (gross) size of the atmospheric CO
2 exchange flux between the atmosphere and the land/ocean are crucial in understanding the carbon cycle and estimating the effects of anthropogenic CO
2 fossil fuel emissions (CO2ff). As the Earth rotates daily and orbits the sun annually, the land and oceanic reservoirs cyclically "inhale" and "exhale" CO
2. These are the exchange fluxes, caused by corresponding changes in temperature, seawater solubility, photosynthesis, plant decay and respiration, resulting in a continuous cycle of absorption and emission. Within this complicated pattern of two-way fluxes, absorption occurs at one geographical location while simultaneously emitting at another. (IPCC Ed., 2001) This paper presents a means of determining the gross exchange flux. Measuring the gross exchange flux is more difficult than the task of finding its nett value, since the gross value does not contribute to the global carbon budget. Current estimates by the Global Carbon Budget (GCB) are for the land exchange flux 130 GtC yr
-1, and for the ocean flux 80 GtC yr
-1 (Friedlingstein et. al., 2022). GCB refers its data source as being IPCC Canadell 2021 (IPCC Ed., 2021) who indicate that for the ocean, the gross figures originate from
Figure 1 of Sarmiento & Gruber 2002 with applied corrections. Earlier GCB reports refer to IPCC (Ed) 2013 which states "Individual gross fluxes and their changes since the beginning of the Industrial Era have typical uncertainties of
more than 20%" (author's highlight).
In the 1970s attempts were made to model the carbon cycle and exchange flux using box models (e.g. Oeschger et al., 1975). Subsequently, General Circulation Models (GCMs) became common, since they could create global geographical distribution maps of the carbon-related processes despite requiring more computer resources. Although GCMs have been helpful in understanding the complexity of the global carbon cycle, box models, with their top-down simplification, offer specific advantages over the open-ended micro-accounting of GCMs. Such simpler models are "better testable" (Popper, 1934) and can help identify errors in formulation by focusing on the most significant factors. This paper presents a 2-box model that computes 14C and 13C isotopic ratios and differs from other box models in several ways. Firstly, its uses atmospheric CO2 levels as input rather than output. Secondly, it employs absolute flow instead of net flow. Lastly, it incorporates a factor describing the proportion of fossil fuel emissions that are directly absorbed by sinks before dilution in the atmosphere takes place. The model accurately calculates 14CO2 and 13CO2 isotopic ratios spanning over 200 years, including the bomb pulse. Yet, notably, it uses the same residence time and reservoir mixing properties for 14C, 13C and 12C.
In addition to uniquely providing estimates of the magnitude of the exchange flux, the method also challenges the widely held view that "
the atmospheric impulse response function of an isotopic perturbation decays much more rapidly than the impulse response function for bulk carbon" (Heimann 1993). See Discussion
Section 5.
1.1. Radiocarbon
The isotopic abundance of
13C and
14C present within atmospheric CO
2 is accurately known globally, with records going back hundreds of years. These isotopes have been measured in samples from tree rings (Stuiver & Quay 1981), ice cores (Rubino et al., 2013), and the atmosphere (Stuiver et al., 1998; D6). While
13C is radioactively stable and forms around one percent of atmospheric carbon,
14C undergoes radioactive decay with a half-life of 5700 ± 30 years (Kutschera 2013), and comprises about 1 part in 10
12. There is virtually no presence of
14C in fossil fuel since it has all radioactively decayed. The
14C/
12C ratio decreased between 1800 and 1960 because of dilution from rising fossil fuel emissions which are
14C depleted, a process known as "Suess dilution" (Suess 1955). In 1960, atmospheric nuclear weapons testing began, causing a doubling of the absolute
14C level within ten years. Since 1975 the level of
14C has decreased (D6), partially due to washout from the exchange flow which contains a somewhat lower level of
14C, but also because of "Suess dilution". Turning now to
13C, its presence in fossil fuels is reduced when compared with the atmosphere, but only by around 2% (Stuiver and Polach, 1977). This small reduction arises from fractionation, which means the larger less mobile isotopic CO
2 molecules are less likely to become embedded in leaves and fossil carbon. Measurements show that the atmospheric
13C/
12C ratio has decreased during the past 200-year period (D4), again partially due to dilution by fossil fuel carbon which contains the lower level of
13C, and partially because of washout from the exchange flow. See
Section 4.2.
2. CO2 Absolute Flow Finite Reservoir Model
The model calculates the movement of CO
2 and its carbon isotopes between the atmosphere and a mixing reservoir, by calibration against isotopic measurements. The size of this mixing reservoir, and the exchange flux are "effective" values, combining the effect of the ocean and terrestrial origins. The concept of effective values is commonplace in the fields of engineering, applied science and economics. For example, economic inflation represents, in a single figure, the average of many different price increases on many items. Productivity measures the effective value created by each hour of work. Effective resistance in electronic engineering or hydraulics gives the overall resistance of a complex system to current or fluid flow. In each of these examples a single value is used to account for a property which is distributed throughout a complex system. The model presented is able to determine the effective values without requiring a detailed audit of the underlying constituent components, unlike other methods. The model consists of two boxes which are considered well-mixed,
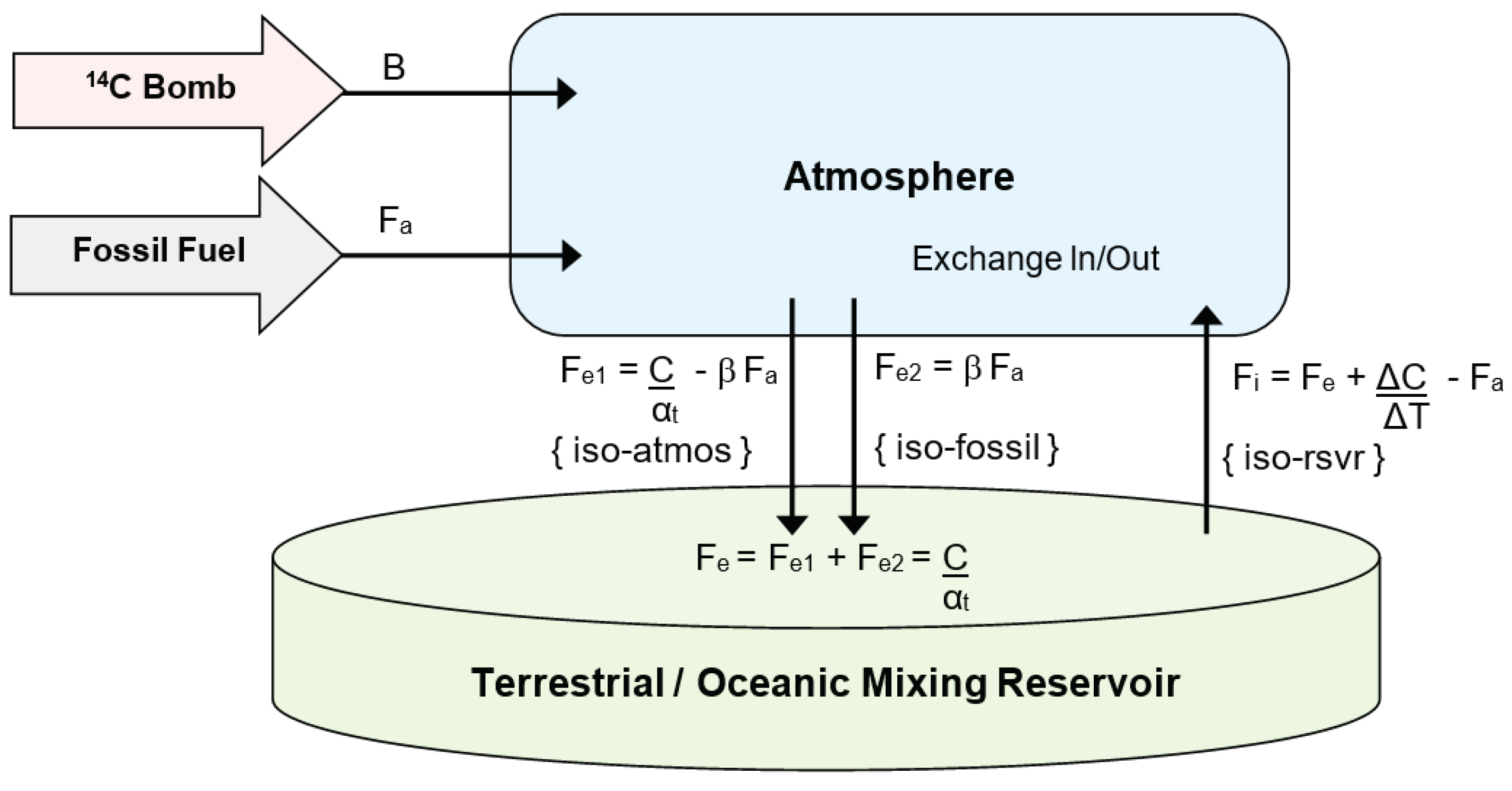
Figure 1. At each annual iteration, the isotopic mixing ratio within the reservoir and atmosphere changes because of incoming
13C and
14C. This change is calculated by the dilution formulae in
Section 2.1. Using a similar notation as Tans et al.(1993) and Cawley (2011) we have
where C is the atmospheric carbon mass, F
a is the anthropogenic input flux from fossil fuel emissions, F
i, is the environmental flux flowing into the atmosphere and F
e is the environmental flux exiting the atmosphere. It is essential that F
i and F
e are kept separate because inflow and outflow are occurring in different places and they are needed to calculate the isotopic flows. Summing these components to form a nett flow would lose track of the separate isotopic mixing occurring across the globe.
Figure 1.
CO2 Finite Reservoir Two-Box Model. The model describes the CO2 flux between the reservoir and atmosphere, including its isotopic carbon contents. Anthropogenic fossil fuel CO2ff Fa, enters the atmosphere and a portion β exits without mixing. The remaining portion, (1-β) mixes into the atmosphere. Otherwise, full isotopic mixing is assumed to occur within each of the two storage regions, atmosphere and reservoir. At each iteration, the CO2 flux outflow, Fe is proportional to atmospheric CO2, C(t); its isotopic level corresponds to a mixture of atmospheric and fossil content. The CO2 influx, Fi is determined by using equation (1) below. Atomic weapons 14C B(t) directly enters the atmosphere with the timing and amounts determined by historical records of individual atmospheric atomic weapons testing, and bomb yield.
Figure 1.
CO2 Finite Reservoir Two-Box Model. The model describes the CO2 flux between the reservoir and atmosphere, including its isotopic carbon contents. Anthropogenic fossil fuel CO2ff Fa, enters the atmosphere and a portion β exits without mixing. The remaining portion, (1-β) mixes into the atmosphere. Otherwise, full isotopic mixing is assumed to occur within each of the two storage regions, atmosphere and reservoir. At each iteration, the CO2 flux outflow, Fe is proportional to atmospheric CO2, C(t); its isotopic level corresponds to a mixture of atmospheric and fossil content. The CO2 influx, Fi is determined by using equation (1) below. Atomic weapons 14C B(t) directly enters the atmosphere with the timing and amounts determined by historical records of individual atmospheric atomic weapons testing, and bomb yield.
We first need an expression which connects the atmospheric CO
2 mass C, to the outflow; F
e is an obvious choice, being
Equation(2) meets the common sense requirements that the absolute exchange exit flux varies in proportion to C, and if C were to be zero, the exit flux, F
e would also be zero. Substituting (2) into (1), we obtain an ordinary differential equation (ODE) for absolute inflow as
The formula also describes the analogous situation of the flow of water from a bucket with a hole of size related to a, and two input taps (faucets) one of which represents the environmental exchange inflow, F
i and the other represents fossil fuel inflow, F
a. In practice, for use with data series, we replace dC/dt with
ΔC/ΔT where
ΔC is the difference of successive carbon atmospheric mass and
ΔT is the time difference, leading directly to the implementation equations shown in
Section 2.4. The formulation differs from those used by other authors in two important respects. First, the incoming flux, F
i is not assumed constant and is calculated at each iteration by "balancing the books". Second, F
e is calculated by direct proportionality to the atmospheric CO
2 mass. As a consequence the model is not a predictive model of CO
2 atmospheric level. However, a great advantage of this formulation is that it enables the inflow to be calculated from measured data, eliminating the need to provide a bottom-up audit of individual CO
2 sources. Furthermore it provides estimates of CO
2 inflow and CO
2 isotopic ratios, and it computes a value for the CO
2 residence time constant. This constant applies equally to all three carbon species
14CO
2,
13CO
2 and
12CO
2. See Section 5.0 for further discussion.
The model uses a bomb yield multiplication factor, Y
b, as one of its seven optimisation parameters, to calculate the nuclear bomb yield of
14C each year, B
14[i]. The bomb yield in megatons is obtained from records of atomic weapons atmospheric test detonations [D5]. A simple time delay of one year was used to allow for atmospheric mixing. The initial values i.e. δ
13C
init, Δ
14C
init, are also optimisation parameters within the model and determine the initial level of the curves in
Figure 3 &
Figure 4, while the parameter δ
13C
FF determines the curve slopes in
Figure 5.
2.1. Mass-Balance and Isotopic Dilution
If a number of gases are mixed but do not react, and each component gas, i, has a mass M
i, then from the law of conservation of mass (also known as Mass Balance)
If within each component gas there is a specific molecule present in a ratio to its mass, R
i, then, since that specific molecule is conserved, the resulting mixture (e.g. paint mixing) having mass M
T has a ratio R
T given by
Equation(4) also applies to the situation of mixing of isotopic gases. For practical reasons associated with historical measurements of radioactivity, studies of isotopic mixtures normally utilize the ratio of the sample activity, A
S to a standard sample activity A
abs, this ratio being known as the relative specific activity, A. Its value is then offset by one to provide the radioactivity scale, δ written (see Strenstrom 2011) in the form
In this scale, although δ increases linearly with As, its value is offset to be zero at the value of the absolute standard (often chosen to approximate background level), as for example in the cases of δ13C and δ14C. Applying (5) and substituting ASi for each component Ri of the mixture, leads after some algebraic manipulation, to
The similarity with equation(4) shows that although δ is defined by an offset ratio scale we can still directly apply equation(6) to calculate the resulting value of δ
T for the mixture. The above scheme forms the basis for the system of equations A
14[], R
14[], A
FF[] and R
FF[] shown in the implementation
Section 2.4 below.
2.2. Fractionation
The model generates values of δ
14C, but we require Δ
14C for comparison with observed Δ
14C values. Δ
14C incorporates a fractionation correction to "translate the measured activity to the activity the sample would have had if it had been wood" (Strenstrom 2011). The correction formulae, which are themselves a function of δ
13C, may be derived from equations by Strenstrom 2011 using equations 25, 28, 38, or Stuiver & Polach 1977 p356, 360,
Table 1, where A
SN is the normalised specific sample activity, A
s is the specific activity of the atmosphere or reservoir, A
abs is the absolute specific standard, δ
13C
M is the
13C sample measurement, and δ
13C
W is the
13C standard for wood, giving
Eliminating A
S , A
abs and A
SN gives
The above formula was used to convert values of δ14C computed by the model to Δ14C. The standard value of δ13C for wood was taken as 25‰, while δ13CM was taken from the model. The correction was small, approximately 10‰ when the bomb pulse has a peak value of 800‰, decreasing to 0.03‰ when δ14C approaches its background value of zero.
2.3. Age Correction
The radioactive decay of 14C over the period from 1820 to 2020 is approximately 2%, since its half-life is 5700 ±30 years (Kutschera, W., 2013). However, this relatively small value is even less significant when one considers that the steady production of stratospheric 14C approximately balances the amount of 14C decay, since the two are in quasi-equilibrium. Therefore, neither 14C decay nor natural stratospheric 14C production were included in the model.
2.4. Implementation
Using equations 1-3 for the amount of CO2 in the atmosphere C, its removal time αt, the atmosphere outflow Fe, the inflow Fi, and the amount of CO2 in the reservoir R, with the square brackets '[i]' referring to the value at each iteration i, we have
Fe[i] = C[i] /αt , Fe2[i] = β Fa[i] ,Fe1[i] = Fe[i] - β Fa[i]
Fi[i] = Fe [i] + C [i] - C [i-1] - Fa [i-1] ,R[i] = R [i-1]+ Fe[i] - Fi[i]
The ratio of 14C to C in the atmosphere is denoted by A14 and in the reservoir by R14 and is calculated as follows. The new amount is given by the previous amount added to the input amounts with the output amount being subtracted, all divided by the total mass (see equation 4). If necessary we include the annual 14C production due to atomic weapons bomb input, B14. Hence we obtain
Similarly, the relative atmospheric and reservoir fossil fuel content AFF and RFF, are calculated on a scale of 0 to 1 using the values at the previous iteration levels, where A refers to the atmosphere, and R the reservoir and subscripts FL means fossil fuel level, and NL means natural non-fossil level,
AFF[i] = (AFF [i-1].C[i-1] + Fi[i-1].RFF [i-1] - Fe1[i-1].AFF[i-1] - Fe2[i-1] + Fa [i-1])/C [i]
RFF[i] = (RFF [i-1].R [i-1] - Fi[i-1].RFF [i-1] +Fe1[i-1].AFF[i-1] + Fe2[i-1]) / R [i]
AFL[i] = C[i] AFF[i], ANL[i] = C[i] - AFF[i]
RFL[i] = R[i] RFF[i] , RNL[i] = R[i] - RFF[i]
The offset isotopic ratiometric measure for
13C is δ
13C and that for
14C is Δ
14C. These are calculated from the iterative series by:-
Initial Conditions: Fi[0] = Fe[0] , A14 [0] = 1 , R14 [0] = 1, AFF[0] = 0 , RFF[0] = 0
It is not necessary to embed a formula for attenuation factor, as discussed by Stuiver & Quay (1981), or for Suess dilution (Suess 1955) because they are implicitly represented.
4. Results
The results are in three main categories; the optimised seven parameters, the isotopic series, and the flux time series.
4.1. Parameter Values
Table 1 lists all seven parameter values along with their estimated errors. The error values were derived by varying each parameter up and down, while keeping the other parameter values constant, until the overall geometric mean standard deviation, σ
Τ approximately doubled. The error figure presented in the table is the mean of the two variations.
1) Residence Time
This compares favourably with Revelle & Suess (1957) of approximately 10 years, and Arnold (1957) of 10-20 years. It has subsequently been stated that "No single lifetime can be defined for CO
2.." (IPCC (Ed.): 2001,
Table 1 p38). This study finds to the contrary.
2) Reservoir Size rel. to Atmosphere (1750)
The value for reservoir size are reported in IPCC Ed., (2013) in GtC as vegetation 450 to 650, soils 1500 to 2400, permafrost 1700, and surface ocean 900, corresponding to a range of 4550 to 5650. This study finds a reservoir size in GtC of 6.18 x 589 = 3640 GtC increasing to 3809 GtC in 2020 with errors of +/- 22%.
3) CO2 Relative Unmixed Uptake
This study determines the quantity of CO2ff entering the sink without mixing in the atmosphere. Factor β accounts for the unmixed uptake, corresponding to that portion of the CO2ff emissions which directly enter the terrestrial/oceanic mixing reservoir. The effect of entry of pure undiluted CO2ff is a lowering of the 14C and 13C curves. The model finds that around 43% of CO2ff exits before mixing while 57% becomes fully mixed. A recent study has revealed that plants near nuclear power stations can uptake significant quantities of 14CO2 (Naegler & Levin 2006). A study of urban grasses near a major highway revealed plants were composed of up to 13% of fossil-fuel carbon (Lichtfouse et al, 2005; Ota et al 2016). According to Kuderer et al (2018), "The 14C signals from such point sources are well detectable in plant samples". Since most fossil fuel emission sources are located near the land or ocean surface, it is reasonable to propose that a significant portion of CO2 fossil fuel emissions are absorbed before fully mixing in the atmosphere.
4) Nuclear Bomb Yield
The model returned a value of 1.6 ± 0.1‰ of
14C per MT for the nuclear bomb yield, with a total bomb yield of 440MT. For comparison, we convert the figures to
14C atoms per MT bomb yield using formulae derived from Svetlik (2010) and Strensom (2011). The weight of carbon atoms, A
CO2 in the atmosphere in 1950, when the CO
2 mixing-ratio was 312.8 ppm, was 664.43 GtC. The number of carbon atoms is given in terms of the molecular weight of naturally abundant carbon, MC = 12.01, and Avogadro's number N
A = 6.022×10
23 by
The
14C/C ratio R
14C can be obtained from the specific activity, α = 0.226 Bq per gram,
14C half life T
1/2 = 5730 years = 1.807 10
11 s, molar weight of
14C is M
C14 = 14, and ln(2) = 0.6931, with
The number of
14C atoms N
14C created is given by the product of the two expressions giving
Hence the number of 14C atoms per MT of bomb yield is 0.0016 x 4.6 x 1028 = 7.4 x 1025, comparing favourably with reported values by Hesshaimer et al. (1994) of 1 to 2 x 1026 atoms per MT. The total bomb yield of 440MT gives a figure of 3.23 x 1028 atoms per MT, which compares with 6.1 x 1028 atoms per MT by Naegler & Levin (2006). The difference may be due to 14C which is stored but does not mix in the biosphere or because of variation in bomb type and design.
5) Isotopic Pre-Industrial Levels and Fossil Fuel Content
a) 14C shows good agreement with Intcal20[D6] for 1820 of 0.7‰. For comparison the maximum level between 1820-2020 is 800‰.
b) 13C initial value, shows excellent agreement with Rubino (2013) 1820 of δ13C = 6.7‰.
c) δ13C for CO2ff is slightly low as compared to figures reported by Stuiver & Polach (1977) for coal of δ13C = -23 ‰.
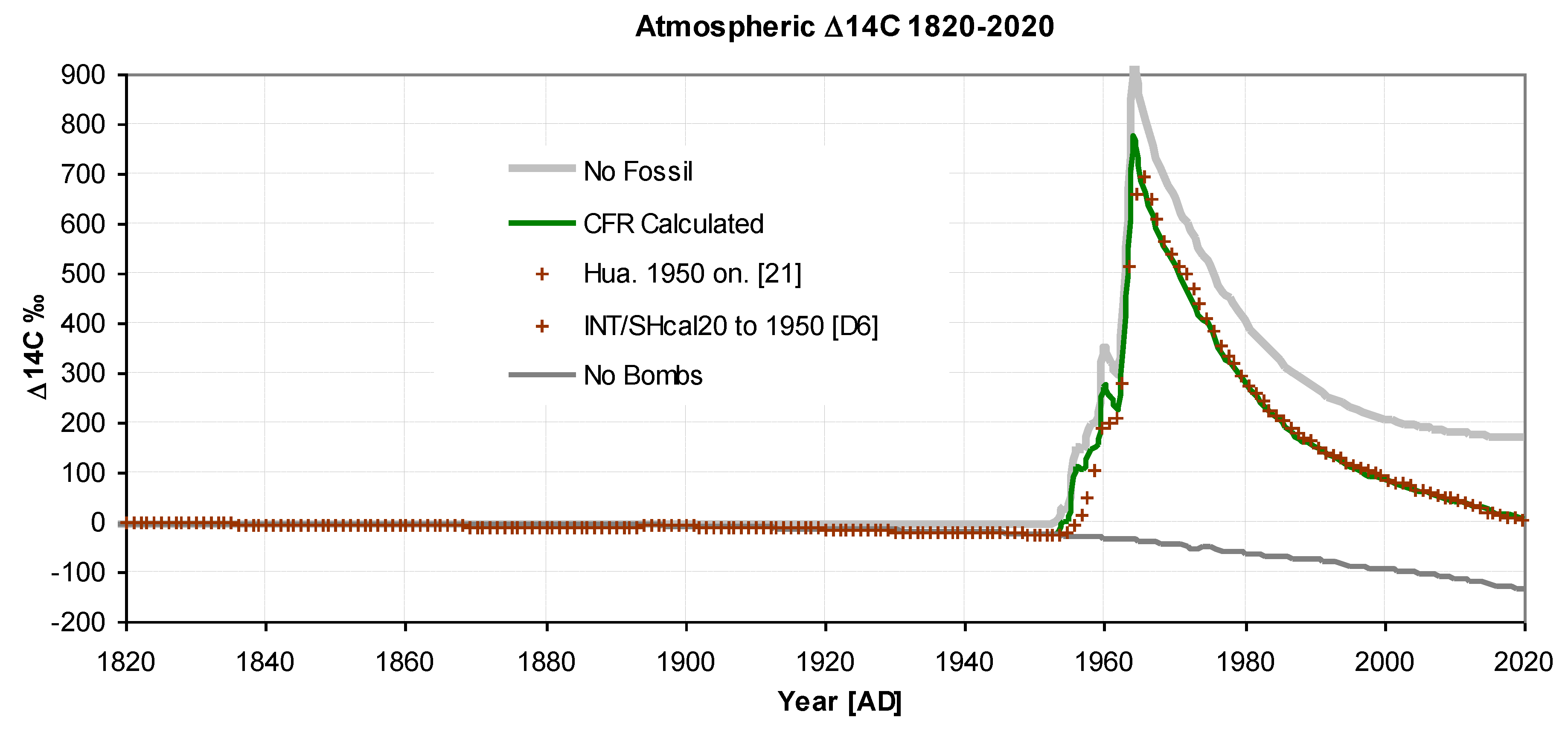
4.2. Isotopic Time Series
Figure 3 &
Figure 4 display the actual and modelled ∆
14C values. The total number of observed data points used was 340, resulting in an overall standard deviation of 0.39‰. The quality of the fit is excellent, with a standard deviation of 3‰, corresponding to 0.4% of the range. In
Figure 3, the slight decrease from 1820 to 1940 is due to Suess dilution, but the magnitude of the dilution is somewhat reduced by reservoir inflow, resulting in excellent agreement with the observations.
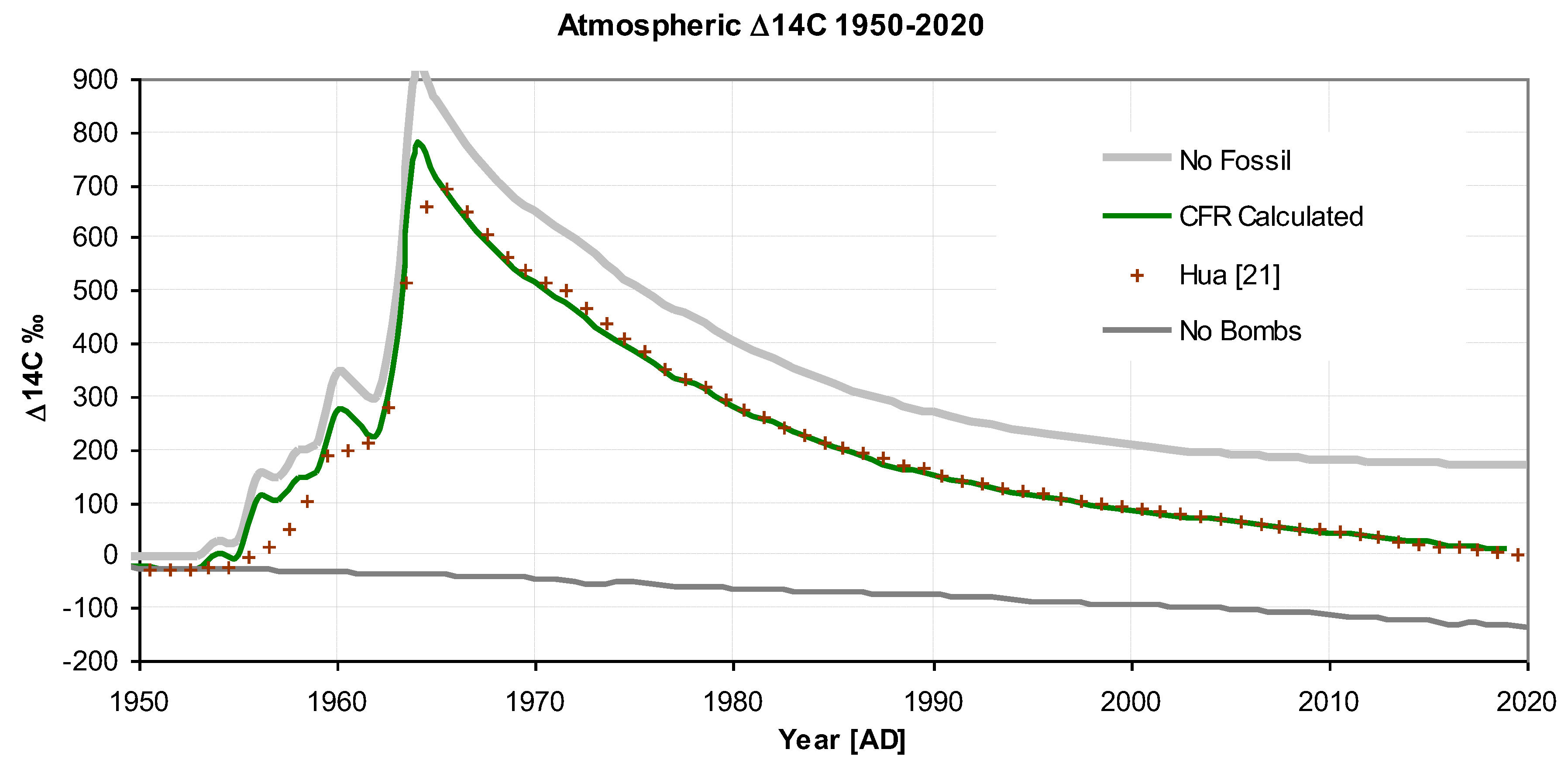
Figure 4 shows the bomb pulse plotted as ∆
14C. Initially, the pulse shape is a decaying exponential but, subsequently, due to Suess dilution and the re-emergence of
14C from the mixing reservoir, the Δ14C decay shape becomes more linear. Despite these complicating factors, the predicted and observed values are in excellent agreement, indicating that the iteration formulae accurately handle the dilution and mixing processes.
Figure 3 and
Figure 4 also show two hypothetical scenarios: "no bombs" and "no fossil". The "no bombs" scenario represents how the decrease would have continued without nuclear atmospheric weapons testing, while "no fossils" shows the situation without fossil fuel emissions. A recent work published by Graven et al. 2020 using a model originally produced by M. Heimann and R. Keeling and rewritten for Matlab, gave very similar results for "no bombs" and "no fossil" scenarios, further vindicating the model presented here and its approach.
Figure 3.
Atmospheric Δ14C 1820-2020. showing 130 values before 1950 [D6] and 70 values from 1968 onwards (Hua et al.. 2022). Standard deviation between observed and predicted is σ = 3.01‰. For "no bombs" and "no fossil" see text.
Figure 3.
Atmospheric Δ14C 1820-2020. showing 130 values before 1950 [D6] and 70 values from 1968 onwards (Hua et al.. 2022). Standard deviation between observed and predicted is σ = 3.01‰. For "no bombs" and "no fossil" see text.
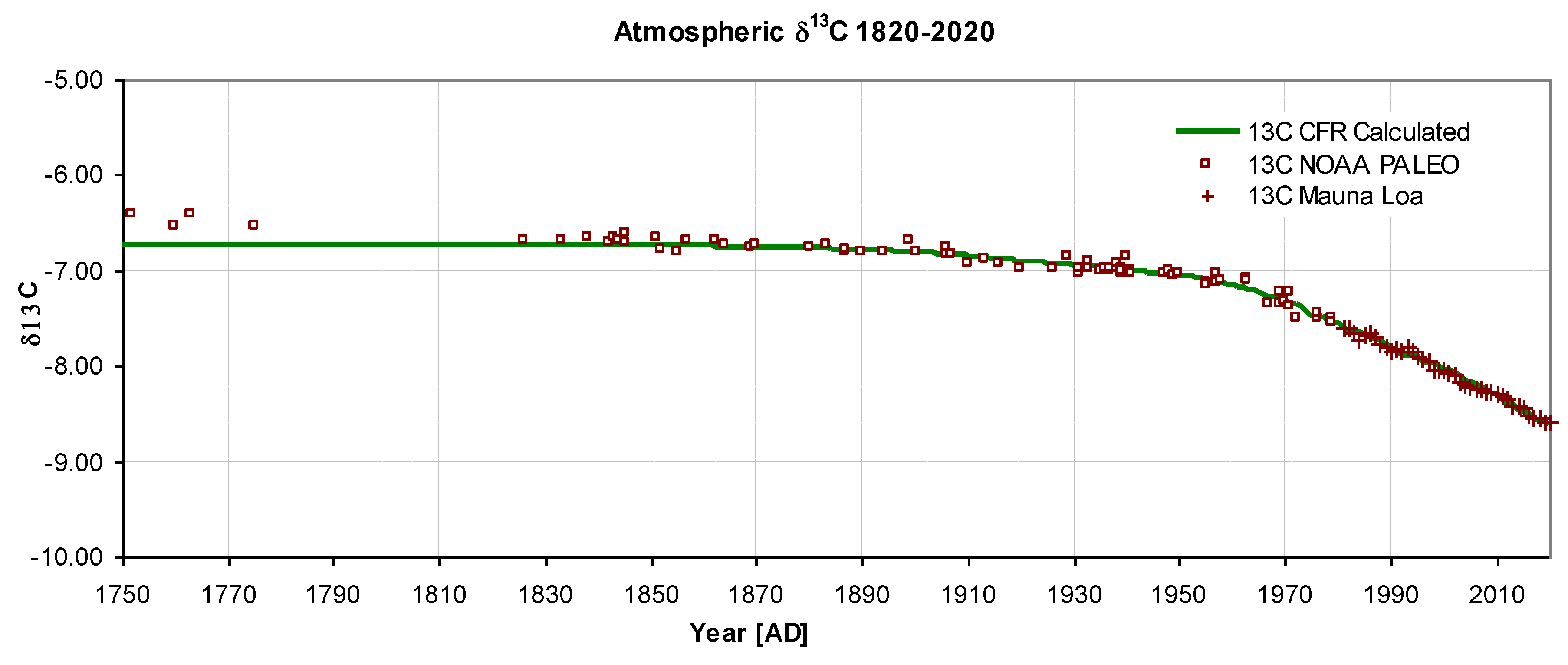
Figure 5 shows the variation of δ
13C over 200 years, with predicted values showing a standard deviation of 0.05‰ from those observed, corresponding to 2.5% of the range. The steady reduction in δ
13C is caused by Suess dilution as fossil fuel emissions contain a lower level of
13C, and by the re-emission of CO
2 from the reservoir. Again, this shape is accurately described by the iteration formulae.
Figure 4.
Atmospheric Δ14C between 1950 and 2020 showing the "bomb pulse" to 2020. For "no bombs" and "no fossil" see text.
Figure 4.
Atmospheric Δ14C between 1950 and 2020 showing the "bomb pulse" to 2020. For "no bombs" and "no fossil" see text.
Figure 5.
Atmospheric δ13C 1820-2020. Model calculated values: solid green, Observed NOAA Paleo [D4]: squares, Mauna Loa [D2]: crosses, σ1820-2020= 0.05‰.
Figure 5.
Atmospheric δ13C 1820-2020. Model calculated values: solid green, Observed NOAA Paleo [D4]: squares, Mauna Loa [D2]: crosses, σ1820-2020= 0.05‰.
Inspection of the curve in
Figure 4 reveals that atmospheric ∆
14C is decreasing to a value below its original background level. This decrease deviates from an exponential decay due to three main factors. a) Incoming fossil fuel emissions (free of
14C) are diluting the atmosphere, this is commonly referred to as "Suess Dilution" (Suess 1955). b) Some of that fossil fuel emissions in the atmosphere is being washed out by the two way exchange flow. c) As time progresses, some of the incoming exchange flow has an increasing level of
14C, because bomb
14C has accumulated in the reservoir and is now being re-introduced to the atmosphere. If the effect (a) were the sole cause of the deviation of the bomb curve shape from a pure exponential decay, the curve would, by now, be well below the initial background. However, effect (b), the washout of fossil fuel emissions, raises the curve. This was noticed many years ago and termed by Stuiver and Quay (1981) the "attenuation factor". Effect (c) raises the tail slightly, further improving the fit in later years to the current level shown in
Figure 4. Keeping track of all of these factors in a "back of the envelope" calculation is complex. The absolute flow model described in this study calculates at each iteration
13C and
14C in the reservoir and atmosphere (see
Section 2.4). It does not need to explicitly consider Suess dilution, the Stuiver attenuation factor or flow-back since these emerge implicitly from the calculation embedded within the implementation equations (2.4)
4.3. Absolute 14CO2, Activity Concentration
The activity concentration of
14C, which is its absolute amount rather than its ratio with C, is displayed in
Figure 6. The graph shows how the activity concentration of
14C in the atmosphere and the reservoir changes over time. The total amount of
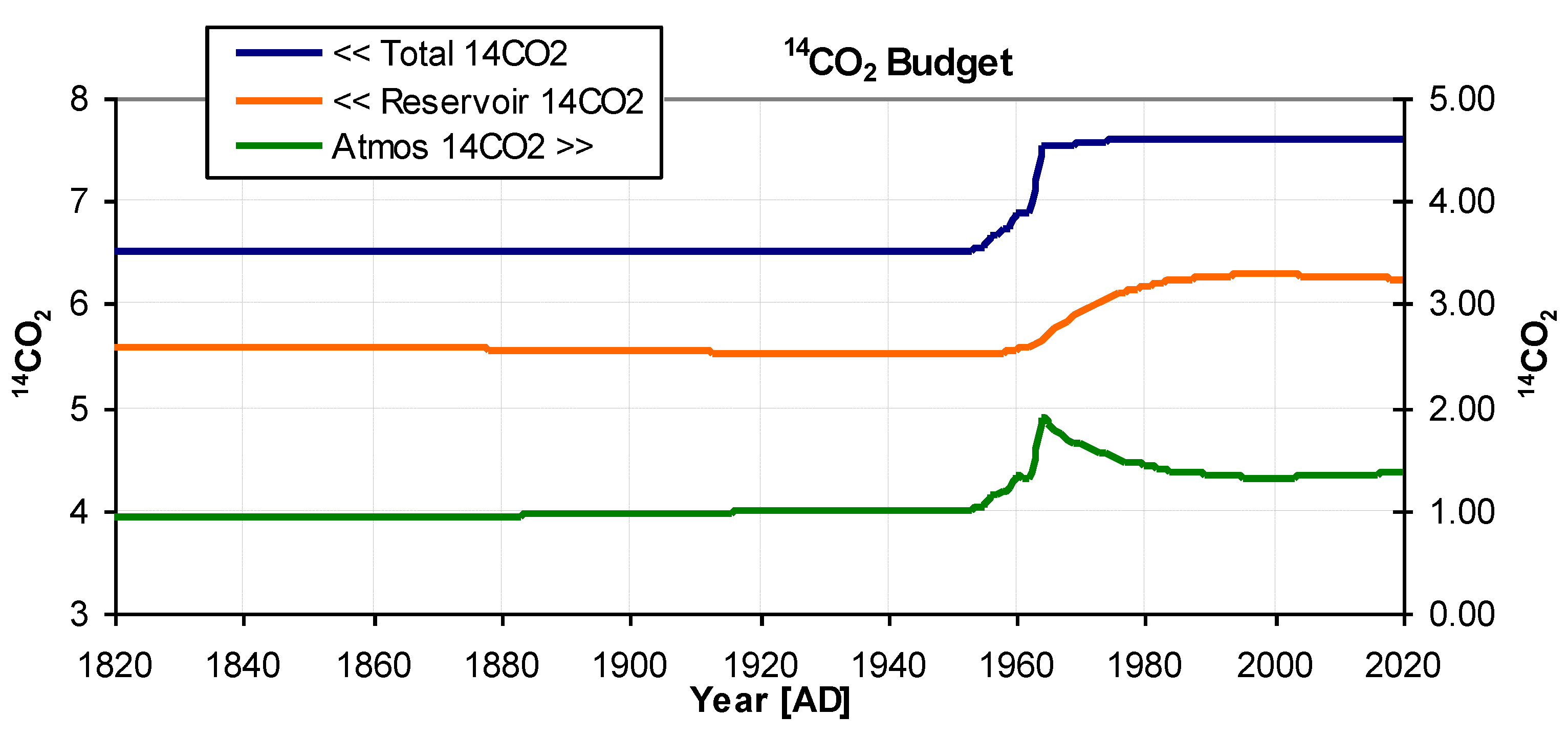
14C in the system is represented by the blue curve, while that in the atmosphere is shown in green. In 1960, atmospheric nuclear weapons testing caused a sudden step increase in the amount of
14C. The atmospheric activity concentration shows the bomb pulse decaying to a minimum around 2000[AD] but then rising slightly again, this has been experimentally determined by Svetlik (2010). Some authors (e.g. Levin et al. 2010) have suggested
14C industrial pollution may be a partial cause of the increase. However, this study accurately reproduces the
14C curve without such a requirement. In this study, the increase in activity concentration can be attributed to
14CO
2 re-emerging from the mixing reservoir, while at the same time causing a slight drop in the reservoir level.
Figure 6.
14CO2 Budget. Model derived absolute 14CO2 (also known as activity concentration) for the reservoir (orange), the atmosphere (green), and their total (blue) in units relative to the 14CO2 content of the atmosphere in 1950. The total, being the sum of reservoir plus atmosphere, shows the near step function in 14CO2 created by nuclear atmospheric weapons testing. After the initial fall of atmospheric content, the small rise since 2000 is confirmed by the results of Svetlik (2010), see text.
Figure 6.
14CO2 Budget. Model derived absolute 14CO2 (also known as activity concentration) for the reservoir (orange), the atmosphere (green), and their total (blue) in units relative to the 14CO2 content of the atmosphere in 1950. The total, being the sum of reservoir plus atmosphere, shows the near step function in 14CO2 created by nuclear atmospheric weapons testing. After the initial fall of atmospheric content, the small rise since 2000 is confirmed by the results of Svetlik (2010), see text.
4.4. Flux Time Series
The model calculates the mean gross exchange flux in 2019 as 60 GtC yr-1, these are approximately 27% of the total IPCC figures (IPCC Ed., 2021 Fig 5.12) of 217 GtC yr-1, and more closely resemble the ocean figures alone of 54 GtC yr-1. Why is there such a discrepancy? We propose here that it is related to the definition of exchange flux. In the real world, if the nett value of a significant two-way exchange flux is zero, it can only have an effect if it has mixed isotopically en route to and from the reservoir. If there is no isotopic mixing, the returned flux is identical to the original absorbed flux both in its bulk and isotopic content; its effect is therefore insignificant. Thus a portion of the exchange flux has no effect. To determine the "effective exchange flux", the model finds the size of the flux which when mixed in both the reservoir and atmosphere, minimises the discrepancy between its computed 13C and 14C curves and the experimentally determined values. This flux is different from the exchange flux of IPCC 2021, which includes cyclical flows of isotopically identical i.e. unmixed gas. This issue also influences the calculation of residence time, which is defined by "atmospheric mass/removal rate", and has been quoted by many in the field (Harvey 2000). The calculation provdes a figure of ~4 years, being obtained by dividing the atmospheric size of 871 GtC by the gross exchange flux of IPCC 2016 i.e. 217 GtC yr-1. However, using the model-determined exchange flux of 60 GtC yr-1, we obtain 871/60 which yields a residence lifetime of ~14 years, similar to the observed decay of the bomb pulse. Because isotopic mixing occurs more readily within the ocean, it is therefore also unsurprising that the exchange figure from the model more closely resembles the ocean exchange figures rather than the terrestrial portion. See Discussion.
4.5. Influx and Temperature
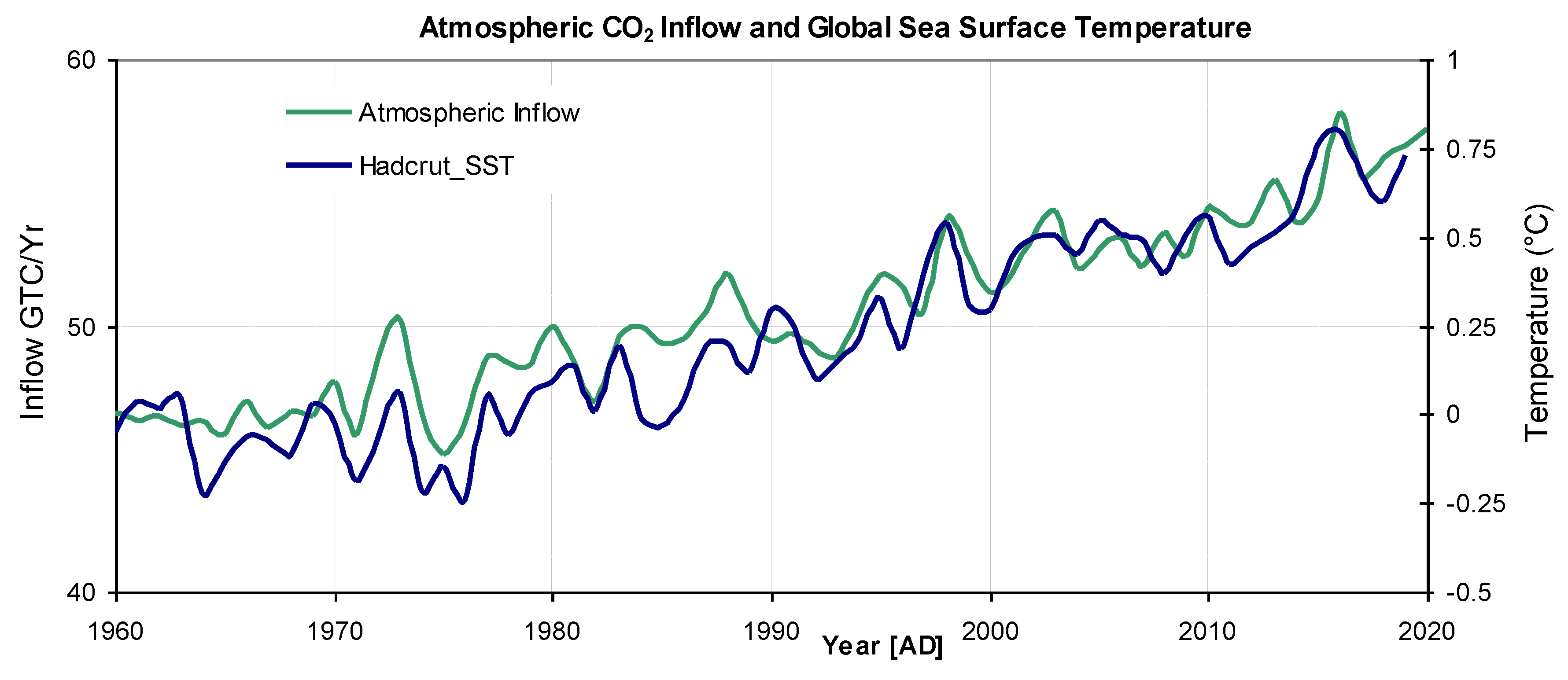
The absolute inflow, computed by the model, is plotted from 1960 to 2020 in
Figure 8. Essentially this is an expanded version of the green plot on
Figure 7. The same graph also shows global temperature (D7) plotted on a suitable vertical scale, revealing that many of the temperature excursions match CO
2 inflow excursions. The correlation coefficient between the two plots is 0.94. Although correlation cannot be considered as causation, it is difficult to conceive of a mechanism whereby a global temperature change could be influenced by the rate of CO
2 inflow, rather than its absolute atmospheric mixing ratio. An obvious temperature-dependant mechanism for CO
2 gas release in the ocean is the decrease of solubility with temperature. On land the balance between productivity, respiration, decay and temerature has been discussed by Melillo et al. 2011. According to the model, from 1960, annual atmospheric inflow has increased by 12.7 GtC yr
-1 while annual outflow has increased by 13.7 GtC yr
-1.
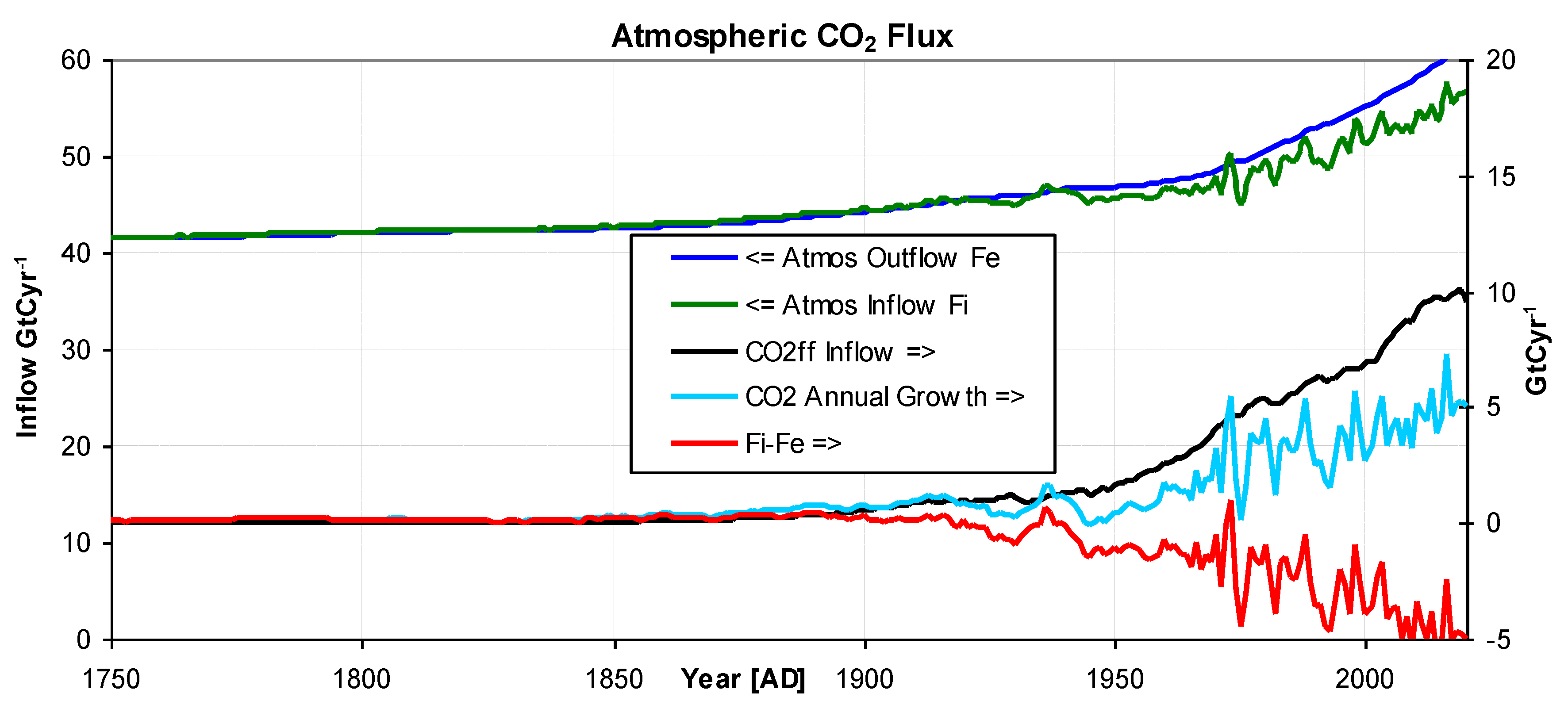
Figure 7.
Output from the model showing:- Atmospheric Outflow (blue), Atmospheric Inflow(green), CO2ff Inflow (black) CO2 Atmospheric growth (light blue), and Fi-Fe, (red). The arrow on the legend indicates the relevant axis.
Figure 7.
Output from the model showing:- Atmospheric Outflow (blue), Atmospheric Inflow(green), CO2ff Inflow (black) CO2 Atmospheric growth (light blue), and Fi-Fe, (red). The arrow on the legend indicates the relevant axis.
Figure 8.
CO2 inflow (green) and observed global sea-surface temperature (blue) indicating a high degree of correlation of 0.94.
Figure 8.
CO2 inflow (green) and observed global sea-surface temperature (blue) indicating a high degree of correlation of 0.94.
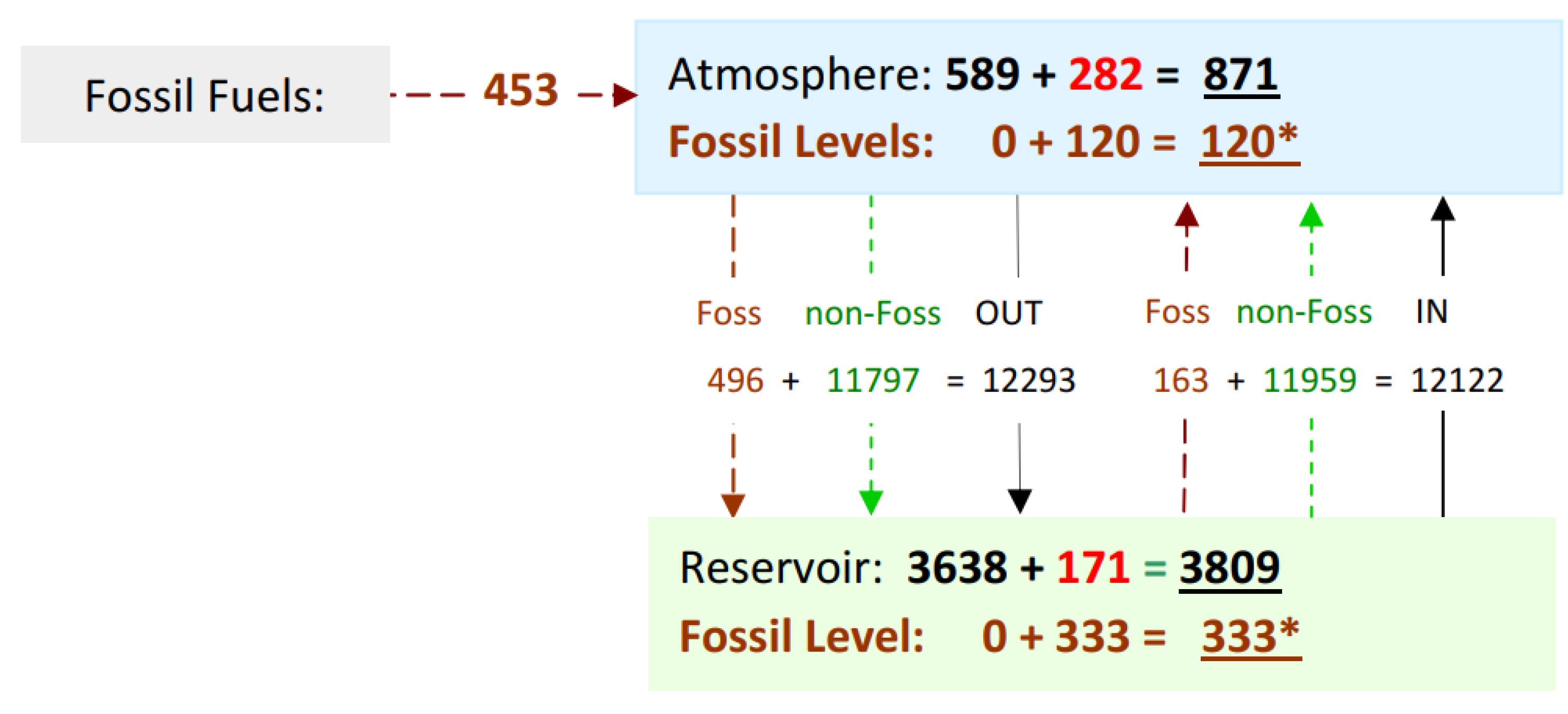
4.6. Cumulative Flux & Levels 1750-2020
Figure 9 shows the cumulative CO
2 flux and CO
2 levels over a period 270 years, during which, 453 GtC of CO2ff was supplied. Out of this, 282 GtC remains in the atmosphere, while 171 GtC is stored in the reservoir. However, the level of fossil sourced CO
2 increased by 120 GtC in the atmosphere and by 333 GtC in the reservoir, also totalling 453 GtC. Although these figures may seem inconsistent, there is no contradiction. The bulk figures indicate the amount of CO
2 without accounting for its isotopic composition. Fossil level is based upon isotopic content, which depends upon dilution caused by the size of the exchange flux in and out and its source and destination isotopic levels. The relative growth of the atmopsheric CO
2 level since 1750 is 48%. The relative level of fossil fuel emmissions within the atmosphere in 2020, isotpically tracked is 14% (See Rice 2022). In terms of bulk flow, there is a net outflux of 171 GtC from the atmosphere.
Figure 7 shows that while both exchange outflow F
e (blue) and inflow F
i (green) are increasing, the outflow has increased more, confirming the nett uptake of CO
2 from the atmosphere by the terrestrial and ocean reservoirs.