3. Results
Optical imaging refers to a variety of noninvasive techniques for monitoring specific physiologic, pathological, and molecular processes and the behaviour of specific cell populations, as presented by Ottobrini [
1] and Pirovano [
2]. These techniques rely on detecting photons emitted by fluorescent and bio/chemo-luminescent molecules in the visible to near-infrared spectrum.
Before discussing several aspects of ICG use in colorectal surgery, a few ideas on the relevance of ICG therapy in cancers are important to mention. The main points of this first section of results involve the relationship between the photosensitivity and stability of ICG and its therapeutic potential in oncology, with reference to research on colorectal cancer.
Photodynamic therapy (PDT) may treat cancer without adverse effects. Indocyanine green (ICG), the only clinically authorized NIR fluorophore, is employed as a PDT photosensitizer. However, ICG's instability in an aqueous solution and poor singlet oxygen (1O2) quantum yield (QY) prevent direct clinical use. ICG-NBs-O2 were formed through hydrophilic-hydrophobic interactions on the gas‒liquid interface from free ICG molecules to enhance PDT effectiveness, as shown in research conducted by Yang[
3]. Another study on the stability of ICG was reported by Lee[
4], where liposomes and phosphatidylcholine-line nanoparticles (PC-NPs) were able to partially stabilize ICG. The authors studied Z/PC-potential NPs (zein phosphatidylcholine hybrid nanoparticles) as an enhanced ICG formulation after discovering that they are a better drug carrier than PC-NPs. These studies show the intriguing potential of Z/PC-NPs as an ICG formulation that stabilizes better than PC-NPs.
Combination treatment may enhance colorectal cancer prognoses due to tumour heterogeneity. Therefore, pH-responsive supramolecular hydrogels based on bortezomib (BTZ) and indocyanine green (ICG) were produced to treat colorectal cancer using photothermal/photodynamic and chemotherapy, as presented by Qing[
5]. BTZ provided pharmacological treatment, while supramolecular hydrogel-wrapped ICG provided photothermal/photodynamic therapy. BTZ and ICG inhibited tumour cells in vitro and in vivo. mPEG-luteolin-BTZ@ICG coupled with laser therapy was biologically safe and offered novel options for advanced colon cancer treatment and valuable references for other tumour treatments. Another study on the possible effects of combination therapy was reported by Ravichandran[
6], who examined nonionic polysorbate-based nanoparticles that delivered piperlongumine (PL) and indocyanine green intracellularly for combined chemo/photothermal/photodynamic cancer treatment (ICG). PL increased oxidative stress in cancer cells to trigger selective apoptosis. ICG is an NIR-absorbing photothermal and photodynamic agent. PL- and ICG-loaded nanomicelles were created using nonionic Tween 80 (T80) polysorbate. PL-ICG-T80 micelles work for NIR light-triggered combination chemo/PTT/PDT.
A study reported by Bi[
7] described a one-pot approach to manufacture tiny lipid-indocyanine green (ICG) nanoparticles (L-ICG NPs) for colorectal cancer therapy. L-ICG NPs were stable, biocompatible, and 20 nm in size. They also cause oxidative/thermal stress damage when exposed to light, which has anticancer effects in vitro and in vivo in HT-29 cells.
In the scientific context of NIR-mediated cancer treatment that has been limited by photothermal drugs' low NIR absorption and photosensitive molecules' poor loading efficiency, Choi[
8] produced a mesoporous silica-coated reduced graphene oxide (rGO) nanocomposite that could encapsulate indocyanine green (ICG) and increase PTT/PDT efficiency in vitro and in vivo. ICG-encapsulated nanocomposites boost photothermal action and produce many tumour-toxic reactive oxygen species (ROS). We found that ICG-encapsulated mesoporous silica (MS)-coated rGO nanocomposite (ICG@MS-rGO-FA) has good colloidal stability and intracellular absorption in folate receptor-expressing CT-26 colorectal cancer cells by conjugating polyethylene glycol (PEG) with folic acid (FA). This ICG@MS-rGO-FA nanocomposite promoted CT-26 cell death through improved PTT and PDT effects after NIR laser irradiation. Normal cells were unharmed. In CT-26 tumour-bearing mice, the ICG@MS-rGO-FA nanocomposite showed good tumour targeting and biocompatibility, improving PTT and PDT in vivo. This tumour-targeted ICG@MS-rGO-FA nanocomposite has remarkable phototherapy potential.
Thermosensitivity in relation to ICG stabilization and combinatorial anticancer therapy was studied by Liu[
9]. An in situ-created photothermal network-based thermosensitive hydrogel (PNT-gel) fabricated from supramolecular cross-linking conjugated polymers stabilizes ICG and provides effective combinatorial photothermal/photodynamic anticancer treatment. NIR photothermal gel-sol transition controlled ICG release. In vitro and in vivo anticancer tests showed that one dose of ICG-loaded PNT-gel administered via intratumoral injection killed cancer cells effectively and nearly completely in combinatorial photothermal/photodynamic treatment under low-dose 808-nm laser irradiation (0.14 W cm -2). Combinational therapy improved photodestruction without tumour recurrence over photothermal therapy (PTT) or photodynamic therapy (PDT) alone. Ren[
10] created a disulfide bond block copolymer to overcome issues regarding the properties of ICG and its therapeutic potential. The polymer employs RGD peptide (ar-arginine-glycine-aspartic acid) as the active targeting group, PEG (polyethylene glycol) as the hydrophilic end, and PCL (polycaprolactone) as the hydrophobic end. To combine chemotherapy with photothermal treatment, we used the amphiphilic polymer to deliver DOC (docetaxel) and ICG (indocyanine green). DOC/ICG-loaded micelles have enhanced in vivo and histological anticancer activity. NIR-guided photothermal treatment enhanced DOC and ICG solubility, and the drug delivery system produced an optimal therapeutic impact. Wu[
11] created a new near-infrared (NIR)-triggered dual-targeted nanoplatform (FA/TPP-DINPs) for mitochondrial combination photothermal chemotherapy by coloading the FDA-approved NIR dye indocyanine green (ICG) and anticancer drug doxorubicin (DOX). The nanoparticles were monodispersed spheres and colloidally stable. Adding the targeted ligands folic acid (FA) and tri-phenylphosphine (TPP) to nanoparticles increased their cellular uptake and mitochondrial colocalization. The FA/TPP-DINPs are a good platform for mitochondrial combination treatment with DOX because the encapsulated dye efficiently converts NIR light into heat. After prolonged 808-nm laser irradiation, the nanoparticles disintegrated due to thermal expansion, releasing DOX rapidly. As predicted, FA/TPP-DINPs increased cytotoxicity and combined treatment efficiency on MCF-7 cells. Thus, the NIR-triggered dual-targeted nanoplatform offers a novel drug delivery approach. Zhou[
12] examined the codelivery of epigallocatechin-3-gallate and diallyl trisulfide by near-infrared light-responsive mesoporous polydopamine nanoparticles for enhanced antitumour efficacy and found that cytotoxicity and apoptosis experiments using this method efficiently inhibited tumour cell proliferation and accelerated cell apoptosis more than diallyl trisulfide or EGCG alone, indicating that the combination of the natural active compounds EGCG and diallyl trisulfide has excellent synergy and can effectively improve the antitumour effect of EGCG.
Regarding the interface between the therapeutic potential of ICG and its use in colorectal surgery in a study by Obinu [
13], the authors found that freshly manufactured poly(ethyl 2-cyanoacrylate) nanoparticles (PECA-NPs) are cytotoxic to cancer cells, making them potential anticancer agents. Indocyanine green (ICG), an amphiphilic tricarbocyanine fluorescent dye, is commonly used in clinical surgery to identify tumoral extensions in diverse organs. This fluorescent substance is unstable and clears quickly in vivo. This work charged ICG in PECA-NPs, increasing its aqueous stability and facilitating tumour cell identification. In vitro interactions between ICG-loaded NPs and tumour cells were examined microscopically and ultrastructurally. The results indicated ICG stability and NP internalization with ICG. TEM showed coated vesicles (< 100 nm) in the cytoplasm, suggesting that they are involved in endocytosis. Thus, ICG-loaded NPs might be used for tumour diagnostics and future treatment.
As shown previously, researchers have created many "all-in-one" nanoplatforms for cancer imaging and PTT/PDT combinational treatment. Their intricate structures, laborious production, use as carriers, and severe side effects limit their biological usefulness. Huang[
14] described nanoplatform (ICG-MB) self-assembly from two FDA-approved dyes, indocyanine green (ICG) and methylene blue (MB), without excipients for cancer fluorescence imaging and combinational PTT/PDT. ICG-MB improved photostability, cellular uptake, and photothermal conversion to substantially ablate cancer cells under 808-nm and 670-nm laser irradiation. ICG-MB accumulated at tumour sites with a near-infrared (NIR) fluorescence signal after intravenous injection, defining the targeted region for NIR laser-triggered phototoxicity. Thus, ICG-MB inhibited tumour development by combining PTT/PDT action without systemic toxicity. This simplistic, effective, and biocompatible nanotheranostic might be a good cancer phototherapy option based on clinical data.
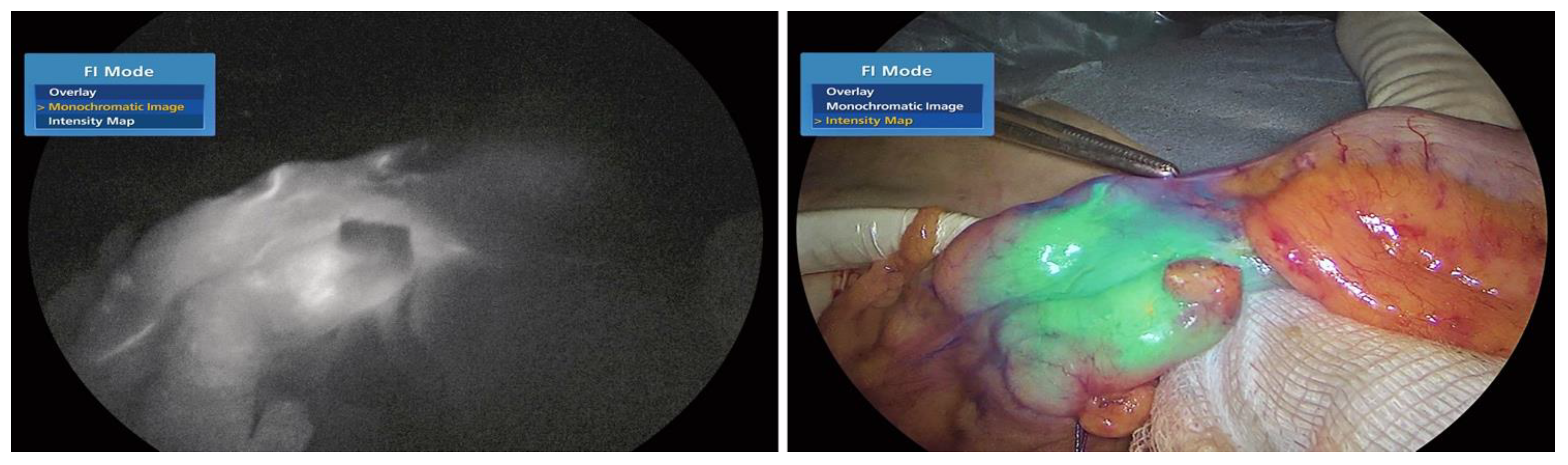
As described by Morales-Conde [
15] in the 2022 guidelines on the use of fluorescence dyes in general surgery, ICG is a fluorescent dye. It is useful for: 1. Better visualization of anatomy (for instance: the biliary tree, the ureters, the parathyroid glands, the thoracic duct) and 2. appreciation of the tissue blood supply (anastomosis in colorectal, oesophageal, gastric, and bariatric surgery and in plastic surgery procedures, liver resection, strangulated hernias and intestinal ischaemia; as shown in
Figure 1a,b); (liver, pancreas, stomach, breast, colon, rectum, oesophagus and skin cancer). The evidence found thus far is encouraging; however, standardization of ICG use and randomized studies with a larger number of patients are necessary to reach definitive conclusions regarding its use in general surgery.
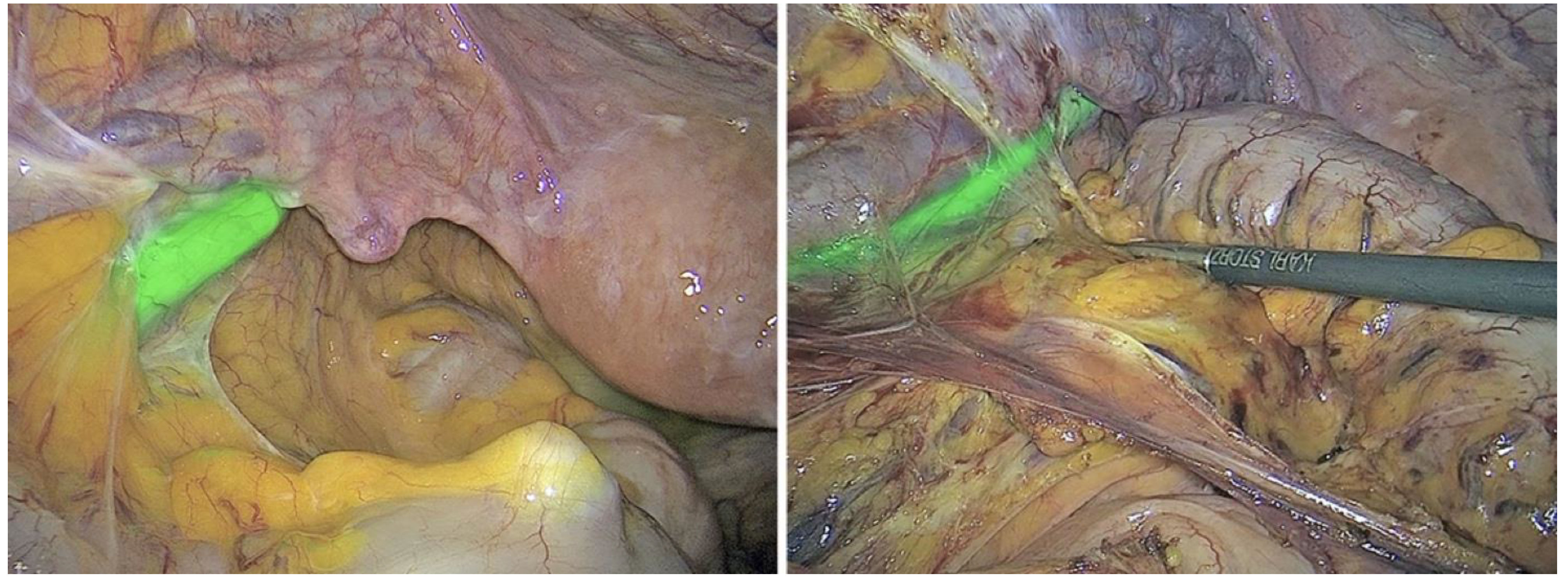
An informed opinion given by Ghuman[
16] on the use of fluorescence in colorectal surgery states that the versatility of this technology improves numerous colorectal procedures. In the past, white light was the only modality for visualizing tissue perfusion, tumour implants, and, importantly, relevant structures such as the ureters and lymph nodes. Because the near-infrared spectrum can penetrate biological tissues, fluorophores can be injected into these structures to identify them. Methylene blue and indocyanine green are the two most commonly used intravenous fluorophores. In addition, new fluorophores specific to tumour markers have been investigated for cancer detection. Thammineedi [
17] called fluorescence-guided cancer surgery (FGS) a new paradigm and focused research on its use in the context of abdominal-thoracic malignancies. Martinez Lopez [
18] described new FGS applications being developed to aid in the detection of peritoneal metastases and the evaluation of tumour resection margins, as demonstrated in
Figure 2a–g.
Heeman [
19] discussed the significance of a standardized protocol for fluorescence imaging and data collection in clinical trials.
Lutken[
20] explored the new quantitative indocyanine green angiography (Q-ICG) technique that provides surgeons with an objective evaluation of tissue perfusion. One thousand two hundred sixteen studies were evaluated, and 13 were included in the final analysis. Maximum and relative maximum intensity could not identify patients with anastomotic leakage. In contrast, inflow parameters (peak time, slope, and t1/2max) more accurately reflected clinical endpoints. Only two studies performed intraoperative Q-ICG, while the rest used video recordings. Research by Noltes [
21] aimed to develop a model of ICG-angiography incorporating standardization and quantification (WISQ) that could be applied even within the surgical novelty domain regardless of the user. The WISQ must be prospectively validated in a larger series before it can be used to predict and prevent postoperative organ dysfunction in a large and diverse surgical population.
A systematic review by Slooter [
22] aimed to identify all methods for quantifying intraoperative fluorescence angiography (FA) of gastrointestinal anastomoses and to identify potential thresholds for predicting patient outcomes, such as anastomotic leakage and necrosis. The quantitative parameters were separated into four categories: the time to fluorescence (twenty studies), contrast-to-background ratio (three studies), pixel intensity (two studies), and numeric classification score (2). The first category was divided into manually measured time (7 studies) and software-generated fluorescence–time curves (13). Cut-off values were derived for manually assessed time (speed in the gastric conduit wall) and derivatives of the fluorescence–time curves (Fmax, T1/2, TR, and slope) to predict patient outcomes. The conclusion was that the time to fluorescence appears to be the most promising metric for determining FA concentrations. Future research could focus on fluorescence–time curves since multiple parameters can be derived and fluorescence intensity persistence can be prevented. To enable future data comparisons, a consensus must be reached on study design, calibration of fluorescence imaging systems, and software validation. A study by Ahn[
23] concluded that since quantitative ICG angiography parameters are affected by various conditions, a standardized protocol is needed. Applying ICG-specific modes at a fixed distance of 4-5 cm can optimize fluorescence images. Several software options have emerged for fluorescence quantification but have not been compared previously. Another study aimed to analyse the results of quantitative ICG-FI assessments of relative perfusion in an experimental setting using two distinct software-based quantification algorithms (FLER and Q-ICG), as presented by Gosvig[
24]. When perfusion was either extremely high or extremely low, the quantitative fluorescence analyses of the two software programs were significantly different. The clinical significance of these variations is uncertain.
Gorspas [
25] FGS offers high sensitivity, contrast, and specificity without exposing patients to radiation. During a surgical procedure, it can also identify the borders and reflect the position of superficial lesions, providing the technical means for early detection and accurate resection of small lesions. Validation of FGS technology's performance through both imaging equipment development and standardization and clinical trials is anticipated to reduce iatrogenic trauma and improve postoperative survival rates and quality of life.
3.1. Recto-Colonic Perfusion
In a study by Gomez-Rosado [
26] designed to objectively evaluate fluorescent signal angiograms with indocyanine green (ICG) in colorectal anastomosis for cancer surgery, the influential factors in its temporary intensity and pattern were identified. In addition, the research assessed the ability to predict ALs (anastomotic leaks) and determined the cut-off levels for high- or low-risk groups. Quantitative analysis of ICG fluorescence during colorectal surgery is safe and feasible for stratifying the AL risk. Hypertension and the site of anastomosis influence the fluorescence intensity at the site of resection (the greatest intensity is detected nearest to the rectum). When fluorescence intensities at the resection site are below 169 U or slopes are below 14.4 U/s, a change in the division site should be considered to prevent AL due to vascular causes. Another study conducted by Mo Son[
27] aimed to find the most accurate predictor of anastomotic complications after laparoscopic colorectal surgery by quantitatively evaluating colon perfusion patterns using indocyanine green (ICG) angiography. Utilization of laparoscopic fluorescence imaging in patients with colorectal cancer. ICG (0.25 mg/kg) was slowly injected into peripheral blood vessels, and the fluorescence intensity of colonic flow was measured using a video analysis and modelling tool to generate perfusion graphs. Patterns of colon perfusion were classified as fast, moderate, or slow based on their fluorescence slope, T1/2MAX, and time ratio (TR = T1/2MAX/TMAX). To identify predictors of anastomotic complications, clinical and quantitative perfusion variables were analysed. Quantitative analysis of ICG perfusion patterns using T1/2MAX and TR can identify segments with inadequate perfusion, thereby reducing anastomotic complications during laparoscopic colorectal surgery.
Nerup [
28] conducted a study to determine whether ICG-FA and q-ICG could improve blood supply evaluations by surgeons with varying levels of training. Thirteen small bowel segments with varying degrees of devascularization and two healthy sham segments were constructed in a porcine model. Blinded to the degree of devascularization, the study recruited students, residents, and surgeons to perform the segments' white light (WL), ICG-FA, and q-ICG perfusion assessments. Compared to standard WL, Q-ICG appears to guide surgeons with all levels of experience to safely perform resections on healthy tissue. The conclusion of the research presented by Tang[
29] was that ICG could effectively reduce the incidence of AL in colorectal surgery without increasing operative time or postoperative complications, a concept also underlined by Vargas[
30]. A meta-analysis by Trastulli [
31] found that ICG-FA administered during colorectal surgery significantly decreased anastomotic leakage and the need for surgical reintervention for anastomotic leaks, particularly in patients with low or ultralow rectal resections. Similarly, sigmoid and rectal resections can have an enhanced AL (lower fistula rates) due to ICG-NIFA technology, as presented by Neddermeyer[
32].
Low anastomoses and preoperative radiotherapy are significant leakage risk factors.
Numerous surgeons perform an unnecessary protective ileostomy, which increases costs and necessitates a second operation for recanalization. A study by Brescia [
33] aimed to evaluate the role of indocyanine green in assessing bowel perfusion, even in the context of a low anastomosis on tissue treated with neoadjuvant radiotherapy. Even in the highest-risk cases, indocyanine green appears to be safe and effective for assessing the perfusion of colorectal anastomoses, potentially reducing the incidence of ileostomy.
The most significant limitation is the lack of a universally replicable standard evaluation. The conclusion of the research by Meijer [
34] was that similar intraoperative fluorescence results might result in different surgical strategies, demonstrating the difficulty of interpreting uncorrected fluorescence signals. Surgeons may soon benefit from the quantification and standardization of real-time NIR fluorescence perfusion imaging. A study by Cheon Kim[
35] underlined that quantitative analyses of indocyanine green fluorescent imaging might help prevent anastomotic complications during robot-assisted sphincter-saving operations and may be especially beneficial in patients with a short descending mesocolon and a high ASA status. A study by Ishii [
36] found that ICG fluorescence angiography can potentially reduce the incidence of AL in laparoscopic rectal cancer surgery patients. Higashijima [
37] evaluated 79 patients who underwent laparoscopic colorectal resection for colon cancer using a double-stapling technique. By measuring the duration of indocyanine green fluorescence, the blood flow in oral stumps was determined (FT). The authors investigated AL cases comprehensively and analysed correlations between FT and AL risk factors. To prevent AL, revision of the anastomosis (stoma di-versification or additional resection) may be necessary for patients with delayed FT (.60 seconds or 50-60 seconds with three risk factors). Aiba[
38] investigated the clinical effect of the time elapsed to arterial perfusion (TAP) on the anastomotic leakage rate (AL), particularly in patients without ICG demarcation, as part of an assessment of blood supply in situations with no clear demarcation line. The employed methods were as follows: The TAP in 110 patients undergoing colorectal surgery was evaluated using ICG-A. For ICG demarcation, the transection line had to be altered, and the TAP was measured at the new stump. The patients were divided into marginal flow (MF) and direct flow (DF) groups based on their arterial routes. The third quartile or slower TAP within each group was determined to be delayed TAP. Conclusions indicated that delayed TAP might be useful for predicting high-risk AL patients in the MF group among patients without ICG demarcation; however, a diverting stoma or strict observation may be a reasonable response.
Numerous solutions, such as ICG angiography and transanal drainage tubes (TDTs), have been proposed to prevent AL. Although the microbiota was only recently recognized to play a role in the pathogenesis of AL based primarily on the results of animal models, whether this mechanism can occur in humans is still unknown. A study by Kawada [
39] analysed this aspect. Insufficient intestinal perfusion necessitating a proximal shift of the transection site was significantly associated with a high faecal volume, which may reflect the correlation between intestinal perfusion and postoperative diarrhoea. In addition, a significant correlation was identified between the intensity of ICG fluorescence at the site of transection and the faecal volume measured by TDT.
Grafitsch [
40] explored visible light spectroscopy (VLS) to evaluate serosal oxygen saturation patterns during colorectal resections. The employed materials and methods included the use of VLS on the colonic serosa and evaluation of the bowel perfusion of patients undergoing left-sided colorectal resections at various times during surgery.
The primary outcome measure was the serosal oxygen saturation (StO2) at various times during surgery at the anastomosis. Researchers observed an increase in colonic StO2 during colorectal resection procedures. The lack of a correlation between AL onset and a decline in StO2 demonstrates the multifactorial nature of AL development. Seeliger [
41] aimed to quantify potential differences in mucosal and serosal perfusion levels in an ischaemic colon segment. Mucosal ischaemia regions were greater than serosal ischaemic areas. These data show that an assessment of intestinal perfusion from the serosal side alone may result in underestimation of the degree of ischaemia. To establish the best resection margin and anastomotic location, further research is needed.
In the field of rectal cancer surgery, high ligation (HL) and low ligation (LL) of the IMA (inferior mesenteric artery) continue to be discussed in terms of perfusion and anastomosis leakage. Han [
42] investigated this aspect using ICG. Randomization was utilized to allocate individuals with rectosigmoid or rectal cancer to the high- or low-risk (LL) group. ICG was administered before and after IMA ligation, and ROI values were evaluated using an image analysis tool (HSL video). F max did not substantially differ between the two groups, although T max and Slope max were different. Significant correlations were found between anastomosis leakage, neoadjuvant chemoradiation, and F max. Following IMA ligation, T max increased and slope max decreased dramatically in the HL group.
Nonetheless, the degree of IMA ligation did not affect perfusion intensity (F max). By assessing blood flow in the remaining colon under fluorescence, Munechika[
43] established the safety and viability of high ligation of the IMA for descending colon cancer without sacrificing the extra distal colon.
AL is perhaps the most difficult complication of anterior resection (AR). Before performing a double-stapling anastomosis, ICG was administered intravenously, and blood flow on the rectal stump was assessed laparoscopically. Iwamoto[
44] examined the connection between several factors and AL. T0—the time between intravenous ICG administration and the disappearance of ICG from the injection route—was considerably longer in the AL group than in the non-AL group (P =.03). No other important disparities were noted between the AL and non-AL groups. Patients with AL had a longer T0 than those without the illness. Only appropriate patients will be able to receive DS if extended T0 can be diagnosed intraoperatively.
Lower rectal cancer with lateral lymph node metastases is considered a local illness in Japan, and lateral lymph node dissection (LLND) is advised. However, laparoscopic operations are relatively difficult. To preserve the autonomic nerve and ureter, the hypogastric fascia and ureter must be detached from the vesical-hypogastric fascia. In addition, lymph node dissection near the internal iliac artery is challenging due to the many branching patterns of the internal iliac artery. Utilizing a near-infrared fluorescence ureteral catheter (NIRFUC) with indocyanine green, Ryu [
45] and Gi-la-Bohorquez [
46] investigated the utility of fluorescence ureter and vessel navigation with ICG. The research concluded that fluorescence navigation of blood vessels and the ureter is possible during laparoscopic LLND and may improve patient safety.
Figure 3a,b depict the use of fluorescent ureteral intraoperative devices for improved identification of the anatomic structures and enhanced and more secure dissection.
In patients with locally advanced rectal cancer, research by Kim[
47] examined the learning process for executing robotic complete mesorectal excision with lateral pelvic node dissection was evaluated using fluorescence.
Subjective visual assessment (SVA) of colour, pulsations, and bleeding from cut edges is inconsistent for predicting conduit perfusion during colon pull-up surgery for corrosive oesophageal strictures. Kumar [
48] identified modest hypoperfusion deficiencies, and ICG-FI may improve the subjective visual evaluation of conduit perfusion. The goal of a study by Spagnolo [
49] was to analyse existing knowledge on the use of fluorescence imaging in intraoperative intestinal evaluations during gynaecological surgery. All studies detailing indocyanine green (ICG) use for bowel assessments in gynaecology or endometriosis surgery were assessed. ICG use was shown to be an effective method for monitoring intestine vascularization, possibly reducing anastomotic leakage and rec-to-vaginal fistula, and is thus valuable for endometriosis surgery and bowel evaluations in gynaecological cancer treatments, a finding also emphasized by Ianieri[
50].
Real-time analysis of the hypovascular pattern of endometriotic nodules correlates with a larger nodule size and lower microvessel density, helping surgeons determine the appropriate transecting line and procedure.
ICG angiography enables laparoscopic and intrarectal examinations of the intestine, which may serve as a double assessment of bowel perfusion and allow evaluations of mucosa vascularization.
Following intestinal anastomosis and discoid resection, ICG fluorescence may guide intraoperative decision-making (also shown by Raimondo[
51]) and rectal shaving, thereby preventing anastomotic leakage and postoperative recto-vaginal fistula in low anterior resections.
3.2. Improving the Detection of Cancer Tissue and Targeted Treatment
Endoscopic imaging is the principal tool for identifying gastrointestinal disorders that are harmful to human health, and a number of advancements and new techniques have been reported by Li[
52].
White light endoscopy (WLE) was the first technique utilized for endoscopic inspection and continues to be the first step in the clinical assessment of gastrointestinal illnesses. Gastrointestinal illnesses are impossible to effectively identify because of their weak connection with histological diagnosis. In recent years, various ingenious endoscopic methods have been developed to improve the detection precision of endoscopy. Chromoendoscopy (CE) boosts the contrast between normal and pathological tissues by using biocompatible dye agents. Narrow band imaging (NBI) may improve the contrast between capillaries and submucosal arteries by altering the light source operating on the tissue using particular filters to view the vascular anatomy. The flexible spectral imaging colour enhancement (FICE) approach employs reflectance spectrum estimation to acquire individual spectral pictures and three chosen spectral images to assemble an improved image of the mucosal surface. Using the varied reflecting qualities of healthy and pathological tissues, i-Scan technology acquires pictures and boosts image contrast using postprocessing algorithms.
In a subgroup of patients with colorectal peritoneal metastases, full cytoreduction has been shown to be the most influential prognostic factor for long-term outcomes.
Fluorescence imaging with indocyanine green appears to be beneficial for finding subclinical peritoneal implants in these individuals. Nonetheless, quantitative fluorescence analysis has not yet been standardized. A study by Gonzales-Abos [
53] investigated the sensitivity and specificity of quantitative indocyanine green fluorescence-q-ICG in the identification of nonmucinous colorectal peritoneal metastases. Intravenous indocyanine green was delivered 12 hours before surgery. By detecting nodules under white light and subsequently with indocyanine green, cytoreduction was performed. Finally, ex vivo fluorescence was assessed. In all, 52 nodules were removed, and histological examination determined that 37 (71.1%) were malignant. Five (13.5%) of the particles were invisible under white light and could only be recognized by fluorescence. Under white light, a total of 15 noncancerous nodules were discovered, 8 of which (53.3%) exhibited negative fluorescence. Fluorescence of more than 181 units may represent the threshold for malignancy, with a sensitivity and specificity of 90.0 and 85.0%, respectively, while uptake of fewer than 100 units tends to correspond to benign pathology. Quantitative indocyanine green use seems to be beneficial for evaluating nonmucinous colorectal peritoneal metastases. Fluorescence uptake exceeding 181 units tends to correspond to malignancy, while fluorescence uptake below 100 units appears to be associated with benign disease. Using postprocessing techniques, technology acquires photos and boosts image contrast.
In a subgroup of patients with colorectal peritoneal metastases, full cytoreduction has been shown to be the most influential prognostic factor for long-term outcomes.
Fluorescence imaging with indocyanine green appears to be beneficial for finding subclinical peritoneal implants in these individuals. Similar findings were reported by Moran [
54]. Lieto[
55] found that the diagnostic performance of ICG-FI was significantly superior to that of both preoperative (p = 0.027) and intraoperative (p = 0.042) conventional procedures, indicating that intraoperative ICG-FI may enhance patient outcomes after CS for PC due to CRC. A study by Lwin [
56] reported fluorescence-associated molecular biomarkers in preclinical and clinical assessments. Numerous fluorescently tagged antibodies, peptides, particles, and other compounds relevant to cancer hallmarks have been produced for the lighting of the target lesion, as underlined by Mieog[
57], Privitera[
58], Nagaya[
59] and Tipirneni[
60]. Applications ranging from fluorescence-guided surgery to endoscopy and tissue biopsy are the focus of research on the topic of recent advances in activable molecular probes, as presented by Zhang [
61].
Even though only a small number of these imaging agents have proceeded beyond early-stage clinical trials, new strategies are being developed to transition them to clinical practice.
For this translational process to be effective, target selection, imaging agents and their accompanying detection systems, and clinical implementation must function in sync to allow real-time intraoperative visualization that benefits patients.
Metal-organic framework-mediated chemo-photothermal therapy guided by photoacoustic imaging (PAI) is a precise and effective method for tumour inhibition that can synergistically induce immunogenic cell death and result in increased infiltration of immune cells into the tumour microenvironment, thereby increasing sensitivity to immune checkpoint blockade (aPD-L1) therapy.
This sort of therapy may not only reduce the systemic toxicity produced by traditional therapies but can also solve the problem of ineffective immune checkpoint blockade's in colorectal cancer (CRC). As described by Liu[
62], to generate multifunctional nanoparticles, the metal-organic structures oxaliplatin (OXA) and indocyanine green (ICG) were loaded into hyaluronic acid (HA)-modified MIL-100 (Fe) nanoparticles (OIMH NPs). The OIMH NPs demonstrated sensitive photoacoustic ageing (PAI) for imaging-guided treatment and a positive synergistic impact when chemotherapy was paired with photothermal therapy (PTT) to destroy tumour cells. Immunogenic cell death (ICD) and T-cell activation caused by chemo-photothermal treatment may sensitize cells to the immune checkpoint blockade response (aPD-L1), therefore inducing systemic antitumour immunity. The combination of chemotherapy, PTT, and aPD-L1 ultimately caused the observed suppression of tumour development.
3.3. Lymph Node Mapping and Lymph Node Metastasis
Widespread use of indocyanine green as a safe and uncomplicated approach for mapping sentinel lymph nodes in different cancer types.
Despite this, indocyanine green has not been extensively applied owing to the disparate results of published investigations. Consequently, the goal of a meta-analysis by Villegas-Tovar [
63] was to assess the overall performance of indocyanine green for sentinel lymph node mapping and node metastases in patients receiving surgery for colorectal cancer. Without time limits, a systematic search was performed to discover relevant English and Spanish research.
For the meta-analysis, hierarchical summary receiver operating characteristic curves (HSROCs) were built, and random effects models were utilized to synthesize quantitative data. Specificity, sensitivity, the positive probability ratio, and the negative probability ratio were determined from each HSROC curve. Indocyanine green for sentinel lymph node mapping is more accurate when applied laparoscopically and to colon cancer according to published findings. However, its overall effectiveness in detecting lymph node metastases is inadequate. A study by Li[
64] explains a technique for fluorescently staining of excised lymph nodes using paired-agent molecular imaging principles, which involve coadministration of a molecular-targeted imaging agent and a suitable control (untargeted) agent, with the signal from the control agent accounting for any nonspecific retention of the targeted agent.
Continuous dual-needle infusion of an antibody-based imaging agent pair (EGFR targeted agent: IRDye-800CW labelled Ce-tuximab; control agent: IRDye-700DX-IgG) or an Affibody-based imaging agent pair (EGFR targeted Affibody® agent: ABY-029; control agent: IRDYN-700DX carboxylate).
Within 22 minutes of tissue processing, identification of a single micrometastasis (0.2 mm in diameter) in a full lymph node could be achieved with >99% sensitivity and >95% specificity.
Existing intraoperative lymph node biopsy methods (e.g., frozen pathology with a micrometastasis sensitivity of 20%) are significantly outperformed in terms of detection capabilities (e.g., frozen pathology with a micrometastasis sensitivity of 20%).
3.4. Inflammatory Bowel Disease (IBD)
An anastomotic leak (AL) after a restorative proctocolectomy and ileal J-pouch increases morbidity and the probability of pouch failure. Therefore, a perfusion evaluation is required during J-pouch development. Although indocyanine green near-infrared fluorescence (ICG-NIRF) has shown the capacity to diminish ALs, its usefulness in restorative proctocolectomy remains questionable. A study by Eder [
65] aimed to develop a standardized method for investigating ICG-NIRF and ALs in pouch surgery. Patients receiving a restorative proctocolectomy with an ileal J-pouch for ulcerative colitis at an IBD-referral clinic were included in a prospective trial, with an AL within 30 days postoperatively as the main endpoint. Intraoperative ICG-NIRF perfusion visualization was performed and filmed at three time points for the postoperative study. The associations between quantitative clinical and technical factors (secondary outcome) and the main result were identified using descriptive analysis and logistic regression. Modifications have been made to the definition and categorization of AL of the J-pouch. To test for AL, pouchoscopy was frequently performed postoperatively. Neither intraoperative ICG NIRF visualization nor postoperative visual analysis revealed AL in 25 individuals. In all instances of ALs, the anastomotic site had a high fluorescence signal and seemed fluorescent (category 2). (4 of 25). The location of anastomosis remained unaltered. ICG-NIRF visualization was consistent and repeatable. The study's findings revealed that visual interpretation of ICG-NIRF may not identify pouch ALs in all situations; quantitative and objective interpretation techniques may be necessary in the future.
A conclusion from research by Spinelli [
66] was that FA might help minimize perfusion-related anastomotic leaks after IPAA surgery, and a prospective, randomized trial is necessary to validate this hypothesis.
3.5. Studies Quantifying Perfusion through the Association with Fluorescence and Other Methods
Traditional approaches for assessing colonic perfusion depend on a visual examination of tissue by the surgeon. Fluorescence angiography yields qualitative data, although interpretation of the obtained signal remains controversial. The correlation between fluorescence and physiological tissue qualities, such as oxygen saturation, is unknown. A study by Soares [
67] investigated a link between fluorescence intensity and intestinal tissue oxygen saturation. The arrival time of indocyanine green in tissues is suggested as a possible tissue oxygenation measurement indicator by Egi [
68], who assessed the tissue oxygen saturation (StO2) in the intestinal tract using near-infrared spectroscopy because it measures oxygen concentrations precisely and presents objective results instantly.
In this investigation, we postulate that the time needed for ICG to reach the anastomotic location after intravenous injection is a useful metric for assessing ICG fluorescence angiography compared to StO2 data from the gastrointestinal tract.
Acute mesenteric ischaemia is a difficult condition commonly caused by intestinal artery obstruction.
Revascularization of a blocked artery and surgical excision of necrotic intestines are included in treatment strategies. In many therapeutic circumstances, necrotic intestines might be difficult to visually recognize. In research by Mehdorn [
69], hyperspectral imaging (HSI) and indocyanine green fluorescence angiography (ICGFA) were found to potentially be effective for the objective intraoperative measurement of intestinal perfusion.
Comparative research was performed according to a concept previously proposed by Barberio [
70] between quantitative fluorescence angiography and hyperspectral imaging for bowel ischaemia assessment. A piece of ischaemic bowel produced by splitting the arcade branches was observed using hyperspectral imaging and fluorescence-based augmented reality. Tissue oxygenation levels were acquired using hyperspectral imaging equipment. Subsequently, fluorescence angiography was performed utilizing a near-infrared laparoscopic camera and 0.2 mg/kg indocyanine green given intravenously. The time-to-peak fluorescence signal was processed using proprietary algorithms to build a perfusion map, which was placed on real-time photos to generate fluorescence-based augmented reality. Nine neighbouring zones of interest were simultaneously selected and superimposed over the real-time footage, resulting in hyperspectral-based augmented reality. The superimposition of fluorescence-based augmented reality and hyperspectral-based augmented reality allowed comparisons of the two imaging modalities. Capillary lactate concentrations were assessed in areas of interest.
Two prediction models employing local capillary lactate levels were extrapolated based on both imaging methods. According to the findings of the research, hyperspectral imaging and fluorescence angiography were employed to assess intestinal perfusion. Hyperspectral imaging yielded more precise findings than fluorescence angiography. Enhanced reality based on hyperspectral imaging may be a beneficial intraoperative method for assessing intestinal ischaemia without the use of contrast.
As poor arterial blood flow and venous congestion both contribute to anastomotic problems, they must be treated concurrently. The objective of a study by Quero[
71] was to evaluate a software-based analysis of fluorescence signals to identify bowel ischaemia patterns. The reported conclusions were as follows: computer-assisted dynamic analysis of fluorescence signal distinguishes between distinct forms of intestinal ischaemia.
Pfahl [
72] quantitatively analysed hyperspectral and ICG data for a more accurate measurement of tissue perfusion. First-ever development of two data processing pipelines capable of recreating an ICG-FA correlating signal from hyperspectral data. On a technical level, the results were analysed and compared to data collected after colorectal resections. In 87% of 46 datasets, the reconstructed pictures matched the original data. The combined use of ICG-FA and HSI within a single imaging system might provide supplementary and complementary information on tissue vascularization, minimize perioperative mortality, and shorten surgical times.
Jansen-Winkeln [
73] found that in 115 patients with colorectal resections, intraoperative HIS (hyperspectral imaging) was safe, repeatable, and not disruptive to the surgical process. Additionally, it assesses the surface perfusion of the gut. HSI might be used as intraoperative guiding equipment, hence reducing postoperative complications.
The greatest constraint in perfusion evaluations using indocyanine green fluorescence angiography during colorectal surgery is that the surgeon subjectively assesses the quality of perfusion. The ideal test for intestinal viability must be objective, minimally invasive, and repeatable. In a study by Kojima[
74], the quantitative value and repeatability of laser speckle contrast imaging for perfusion assessments during colorectal surgery were evaluated. Laser speckle contrast imaging is viable for real-time, quantitative, and highly reproducible evaluations of intestinal perfusion during colorectal surgery without the use of contrast agents according to the study's findings. In contrast, Jonas Hedelund Ronn[
75] found that Q-ICG and LSCI (laser spectrum contrast imaging) are not interchangeable but may be complementary. Angle and distance have a considerable impact on LSCI. In contrast, q-ICG is slightly impacted by shifting experimental circumstances and is more easily used with minimally invasive techniques.
Joosten [
76] investigated the use of ICG in an acute setting and reported that intraoperative use of FA alters surgical choices for intestine resection for intestinal ischaemia, possibly permitting gut preservation in one-fourth of patients. To optimize the best use of this technology for this indication and to establish standards for the interpretation of FA images and the potential need for second-look surgeries, prospective studies are needed.
Vaassen [
77] developed algorithms for automatic extraction of inflow-based parameters, including the time to reach 50% of maximal intensity (T (1/2) and the maximal normalized slope (slp n). These parameters provide a more accurate representation of clinical endpoints than direct intensity measurements. The generation of immersive cartograms and direct access to objective information regarding bowel viability after vascular reconstruction is made possible by an automated method. The aim of research by Park[
78] was to evaluate the ability of AIRAM to predict the anastomotic complication risk in patients undergoing laparoscopic colorectal cancer surgery. When the ICG graph pattern exhibited a stepped rise, the accuracy of conventional quantitative parameters declined, whereas the AI-based classification consistently maintained its accuracy. The analysis improved statistical performance verification. D'Urso [
79] researched fluorescence-based enhanced reality (FLER), a computer-based quantification method for evaluating bowel perfusion using fluorescence angiographies. The purpose of this prospective study was to assess clinical feasibility and correlate FLER with metabolic perfusion markers during colorectal resections. FLER enables visualization of the quantified fluorescence signal in augmented reality and offers a reproducible estimation of bowel performance.
The goal of a study by Tokunaga [
80] was to objectively measure intestinal temperature with the aid of thermography, which can be helpful for assessing blood perfusion. Temperature measurement by thermography is a useful new method for assessing intestinal blood perfusion. Using thermographic images, the temperature boundary can be easily identified. The residual intestinal tract temperature was significantly higher than that of the resected intestinal tract at the planned separation line.
3.6. Estimation of Optimal Resection in Relation to Liver Function for Colorectal Metastases
ICG’s primary use in visceral surgery is to evaluate the perfusion of gastrointestinal anastomoses and to promote lymph node dissection. During staging laparoscopies or cytoreductive surgery, it may potentially be utilized to identify liver metastases, as shown by Knospe [
81].
Patients undergoing hepatic surgery have traditionally been examined utilizing radiological and quantitative measurements of liver function. Considering the increasingly difficult and lengthy nature of current surgical techniques, usually in the context of cirrhosis/fibrosis or following chemotherapy administration, whether extra examinations may be required prior to performing such surgeries remains in question. The goal of a review by Morris-Stiff [
82] was to characterize the present level of knowledge on pre- and postoperative quantitative assessments of hepatic function in patients undergoing hepatectomy and liver transplantation. Indocyanine green clearance is the most popular approach for dynamically measuring hepatic function, as determined by the review. Performing a large hepatectomy on individuals with minimal hepatic function is challenging. Due to the likelihood of postoperative hepatic failure, surgery is contraindicated in some cases. In a case report by Kato [
83], the first patient had limited liver function (ICGR15: 28%) and was successfully treated with a right hepatectomy, which allowed preservation of the complete caudate lobe. All metastatic lesions shrank after chemotherapy; however, the patient’s ICGR15 and ICGK were 21% and 0.12%, respectively. The remaining liver volume was only 39%, indicating portal vein embolism (PVE) in the right portal vein. Portography revealed significant preservation of the right caudate lobe branch (PV1R) from the right portal vein's origin. After 18 days, liver function was re-evaluated. During this period, the ICGR15 (21–28 percent) and ICGK rates (0.12–0.10 percent) declined. The conclusion was that the procedure was a safe and viable surgical method for patients with marginal liver function.
A study by Li [
84] determined the relationship between the intensity of indocyanine green (ICG) fluorescence during near-infrared fluorescence-guided surgery (NIRFGS) and preoperative liver function markers. The absolute fluorescence intensity in liver damage mouse models was greater than that in control animals. The rate of ICG removal from the tumour in the liver damage model group was equal to that in normal mice. However, the clearance rate of the background was slower than that of normal mice, increasing the ideal period for the tumour-to-background ratio (TBR). In addition, correlation analysis was used to evaluate which preoperative liver function measures had the strongest correlations with hepatic ICG clearance. Liver damage has no substantial influence on the maximal TBR, but it lengthens the optimum TBR period, thus providing a larger and more stable surgical window. Using NIR fluorescence imaging technology for preoperative liver function tests, this research indicated that a delayed surgical start time is achievable.
3.7. Flap Assessment
For the treatment and restoration of complicated perineal fistulas, gracilis muscle interposition (GMI) is a well-established approach. Limiting the results are complications such as necrosis, poor wound healing, and fistula persistence or recurrence. No intraoperative quantitative techniques for assessing the perfusion of muscle flaps are available. Research by Lobbes [
85] analysed a novel and objective software-based assessment of ICG-NIRF in GMI. Five patients with inflammatory bowel disease undergoing GMI for perineal fistula and reconstruction had their intraoperative ICG-NIRF visualization data analysed retrospectively. The new software was utilized to generate perfusion curves for the specific regions of interest (ROIs) of each GMI. These novel perfusion indicators (curve shape, maximum slope value, distribution, and range) indicated adequate perfusion.
3.8. Experimental Research and Results Foreshadow Future Clinical Achievements
Decreased intestinal perfusion is believed to contribute to the pathogenesis of necrotizing enterocolitis (NEC). Research by Knudsen [
86] was designed to examine intestinal perfusion in NEC lesions. During laparoscopic and open surgery, quantitative fluorescence angiography using indocyanine green (q-ICG) was performed. To develop neonatal enterocolitis (NEC), 34 preterm piglets were born through caesarean section and given parenteral nourishment and increasing quantities of infant formula. During surgery, macroscopic NEC lesions were examined using a validated macroscopic grading method (1–6 for increasing NEC severity). Using q-ICG and a proven computer technique for pixel intensity, intestinal perfusion was assessed and quantified. The conclusion was that q-ICG seems to be a realistic and helpful approach for measuring tissue perfusion in NEC lesions.
Recently, neurons were found to generate near-ultraviolet (NUV) light fluorescence. The objectives of a study by Dip [
87] were to establish the extent to which nerves glow brighter than background and vascular structures under NUV light and the intensity of NUV light at which nerves are most identifiable from other tissues. At all NUV intensities, a fluorescence score of 200 showed 100% accuracy for distinguishing nerves from other anatomical structures in vivo. In animal model experiments, nerves were analysed by determining the ratio of nerve signals to background, as reported by Barth[
88].
Wang [
89] demonstrated how a novel cyclic TMTP1 homodimer TMTP1-PEG4 ICG was successfully constructed and synthesized, which exhibited more sensitive tumour detection than its monomer. It was also able to distinguish lymph node metastases from normal lymph nodes and was demonstrated to be an attractive photothermal agent that significantly inhibited tumour growth when exposed to NIR laser light. In addition, extremely porous and injectable hydrogels generated from cartilage acellularized matrix demonstrated dual responsiveness to NIR light in antitumour treatment, as demonstrated by Gulfam[
90].
Current research investigates multispectral analyses of dyes to improve the precision of the findings. For instance, Polom [
91] used a single-camera system for two separate near-infrared wavelengths and deployed two fluorophores—indocyanine green (ICG) and methylene blue (MB)—throughout various phases of colorectal surgery. The research uncovered a substantial possibility for the use of two separate fluorophores in colorectal surgery, where the visualization of one fluorophore did not interfere with the quantitative analysis of the other fluorophore. Utilizing two separate dyes during a single treatment may help improve the fluorescence characteristics of both dyes for their respective uses. Visualization of distinct structures by different fluorophores appears to be the future of image-guided surgery and represents advancements in the optical technologies of image-guided surgery. Van Beurden[
92] emphasized that future problems will be presented by the integration of complementary fluorescent readouts during the same surgical process, commonly known as multiwavelength fluorescence guidance.
Although laparoscopic resection for early gastric and colorectal cancers is becoming increasingly common, the loss of touch sensation complicates identification of tumours in the stomach and intestine. Lee[
93] proposed the use of indocyanine green (ICG)-loaded alginate hydrogel as a fluorescent surgical marker for correct laparoscopic operations. The results presented were as follows: the ideal concentration of the ICG-HSA complex was found to be 30 M, and the greatest fluorescence intensity was attained with a mole ratio of 1:1 between HSA and ICG. Subcutaneously injecting ICG or ICG-HSA solution into mice caused the fluorescence signal to diffuse quickly, surrounding the injection site within 3 hours, and 24 hours later, faint fluorescence was identified around the injection site. In contrast, ICG-HSA-loaded alginate gel successfully extended the fluorescence detection period up to 96 hours postinjection, inhibiting the injection-site dispersion of the injected ICG. During laparoscopic surgery, injection locations of the hydrogel in the porcine stomach could be properly identified in real time even after three days. This alginate hydrogel technique is quite effective in providing an accurate and durable surgical marker for laparoscopic procedures.
The objective of a study by Marston[
94] was to identify an optical imaging agent for use with FGS technology in CRC. The authors compared a panitumumab-IRDye800CW conjugate to an isotype control IgG-IRDye800CW conjugate. Mice were implanted with one of three CRC cell lines (LS174T, Colo205, and SW948), and pictures were captured using open- and closed-field fluorescence imaging systems. To measure fluorescent contrast, the ratio of tumour fluorescence to background fluorescence was computed. After 10 days, the mice were slaughtered, and their tumours were stained for microscopic examination. In a murine model of colorectal cancer, Pani-tumumab-IRDye800CW generated considerably stronger fluorescence contrast than IgG-IRDye800CW and was appropriate for the use of FGS technology in CRC.
Optical imaging (OI) delivers real-time clinical imaging and concurrent genetic, morphological, and functional data on disease processes.
Kan[
95] demonstrated a unique interventional OI approach that enables in vivo visualization of three separate pathologic zones of ablated tumour periphery for prompt identification of residual tumours during radiofrequency ablation (RFA) therapy. Eight rabbits with orthotopic hepatic tumours were divided into two categories: partial RFA and full RFA. Interventional OI based on indocyanine green was used to differentiate between three pathological zones: ablated tumour, transition margin, and residual tumour or surrounding normal liver, with quantitative comparisons of signal-to-background ratios between the three zones and between incompletely and completely ablated tumours were performed. Ex vivo OI and its correlation with pathology were used to validate the results of interventional OI. Interventional OI could distinguish between partly and totally ablated tumour margins, which allowed residual tumour identification. This technique may provide new opportunities for evaluating tumour eradication during a single interventional ablation session.
During a stress reaction, brain neuropeptides are produced in a spatially and temporally coordinated manner. For precise knowledge of the roles of peptides during a stress response, their release, diffusion, and breakdown in the brain must be examined.
Indicators of genetically encoded fluorescent calcium have significantly advanced our understanding of the functions of specific neuronal activity in the regulation of behavioural changes and physiological responses during stress over the past two decades. Furthermore, numerous structural details regarding G-protein-coupled receptors (GPCRs) for neuropeptides have been discovered. OT is generated by enteric neurons, and both enteric neurons and enterocytes display developmentally controlled OT receptors, which is related to the link between OT and neuronal growth at the gut level (OTRs), as found by Welch [
96] and Padurariu [
97]. The results indicate that OTR-mediated signalling is a crucial physiological regulator of enteric neuronal activity, mucosal homeostasis, intestinal permeability, and intestinal inflammation.
Bergenheim [
98] and Raducanu[
99] found that as part of the homeostatic process, intestinal stem cells found at the base of Lieberkühn crypts produce offspring that replace resident cells lost from the tip of the villi.
These stem cells may be generated in vitro as organoids, and orthotopic transplantation in mouse models of mucosal injury demonstrated that intestinal organoids could spontaneously connect and integrate into the injured epithelium, therefore accelerating healing and resulting in increased weight gain.
This finding suggests that transplantation of intestinal stem cells may be applicable in humans to actively promote mucosal healing and may be employed to treat a broad spectrum of gastrointestinal disorders, including inflammatory bowel disease, where mucosal healing is a key treatment objective and the most significant predictor of clinical remission.
Following transplanted cells in vivo is crucial, particularly during the preclinical period, to evaluate engraftment effectiveness and monitor wound healing.
The practicality of labelling intestinal organoids with a panel of fluorescent dyes and nanoparticles for visualization with a clinically authorized imaging modality—confocal laser endomicroscopy—was examined (CLE).
The homogeneity, durability, cell viability, differentiation capability, and efficiency of organoid creation were evaluated by CLE together with in vitro and ex vivo observation of labelled organoids.
Fluorescent dye-based tagging can be utilized in tandem with CLE to follow intestinal organoids after transplantation to confirm implantation at the intestinal target location.
Theoretically, any antibody or tiny cancer-targeting molecule might be tagged with bioluminescent or fluorescent substances. Fluorescence imaging (FI) and bioluminescence imaging (BLI) have been widely used in preclinical research to assess tumour volume, evaluate the targeting of tumours by experimental medicines, and differentiate the main effects from the secondary effects of cancer therapy. The development of highly cancer-specific fluorescent probes that can be visualized is currently underway, which will allow staging of malignancies and monitoring of innovative treatment agents and facilitate proper surgical resection and image-guided biopsies. According to research by Woo[
100], (1) fluorescent proteins that are physiologically safe, stable, and clearly visible with a high target-to-background ratio and 2) extremely sensitive optical detectors are crucial FI components.
Present research accomplishments have been reported by De Galitiis[
101] in p53 gene mutations in colorectal cancer by means of fluorescence-assisted mismatch analysis (FAMA). Thorough research on p53 gene polymorphism in a specified population might be conducted using FAMA by focusing on a specific location (based on a geodemographic classification, such as Romanians and their genetic polymorphism, as described previously by Murarasu[
102] [
103]). Because the discovery of more frequent mutations might indicate earlier detection and treatment, such an intervention may consistently contribute to cancer control in that region.
In addition, q-ICG might potentially be utilized to diagnose and treat a localized infection in the abdominal area, such as either an intraperitoneal (Xie [
104]) or retroperitoneal site (Marincas [
105]).