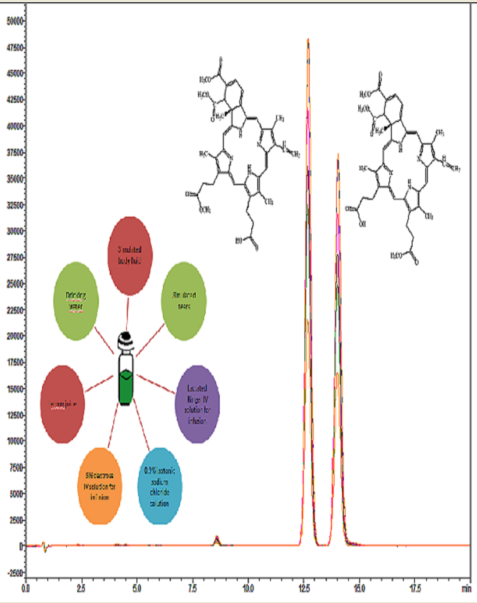
The aim of this study, for determination of verteporfin in real samples (simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water) was the method validation and to examined by HPLC-DAD-UV. Metod validation parameters such as specificity, linearity, precision, accuracy, robustness, limit of detection (LOD) and limit of quantitation (LOQ) for verteporfin were validated and developed according to the International Conference on Harmonization (ICH) Q2 R1 guidelines. The LOD and LOQ for verteporfin were found 0.06 µg/L and 0.2 µg/L, respectively. The recovery values of the optimization and validation for verteporfin were found in the range of 97.5-100.7%. The relative standard deviations (RSD) for vertepofin were <1%. The developed method was successfully applied to real samples with high accuracy and the recoveries (%) from real samples were 99.9, 100, 98.2, 99.2, 99.4, 98.8 and 99.4, respectively.