1. Introduction
One of the most widely used models to predict membrane fouling is the Hermia model [
1]. In the 1982 paper, Hermia was able to frame mathematically the relationship between the accumulated volume and time from experimental data, arriving at the differential equation presented in Eq. 1. Since this model was derived for non-Newtonian fluids, the parameters
n and
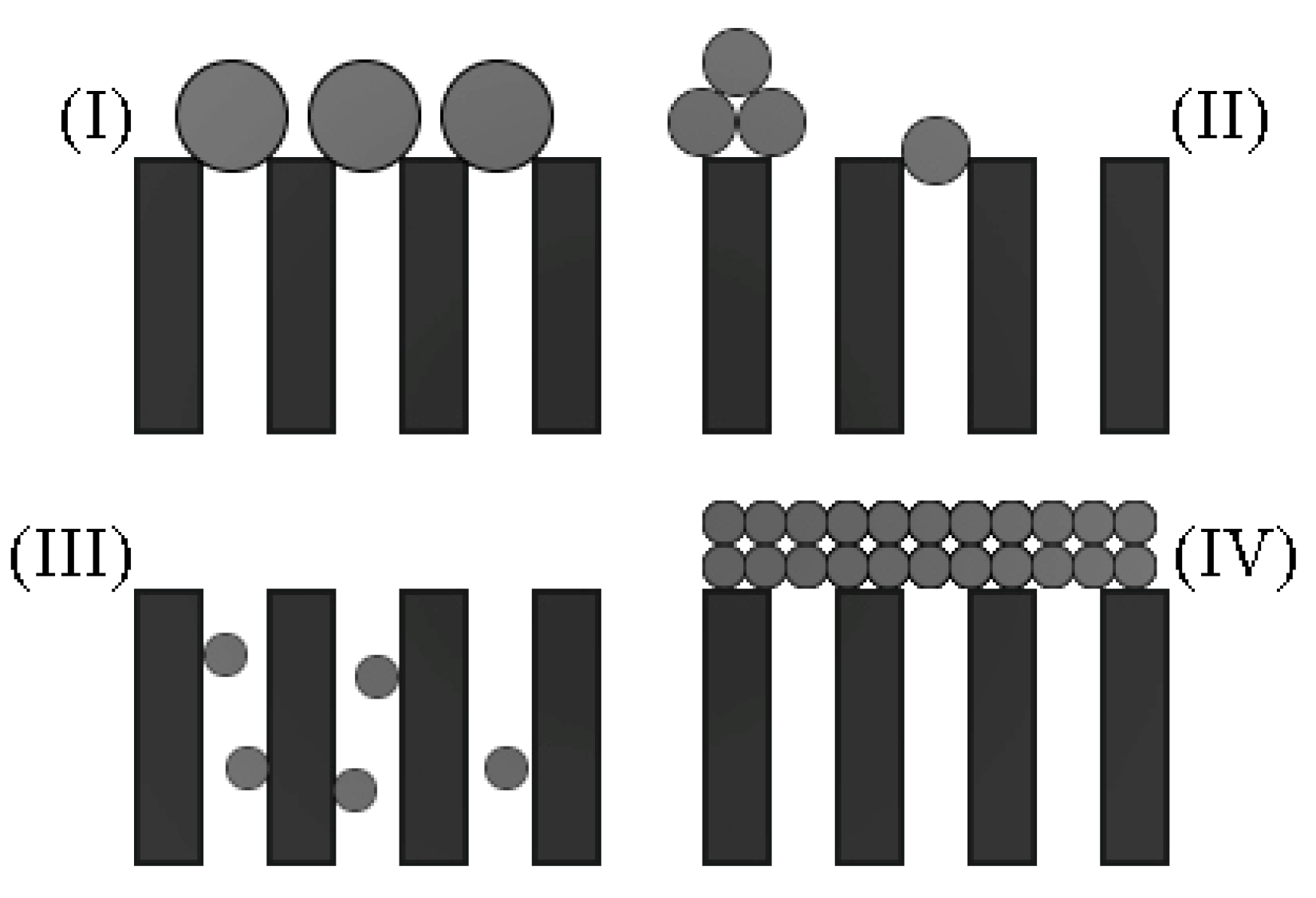
k help to adjust the model for different types of fluids and blocking mechanisms. The original ordinary differential equation (ODE) was solved for four different discrete values of
, each value with its blocking mechanism, as shown in
Figure 1 and Equations 1-4.
| Complete blocking (CB) () |
|
Eq. 2 |
| Intermediate blocking (IB) () |
|
Eq. 3 |
| Standard blocking (SB) ( |
|
Eq. 4 |
| Cake formation (CF)() |
|
Eq. 5 |
Where
is the time measured from the beginning of the filtration experiment,
is the permeate flux at time
,
is the permeate flux at time
and
is a real constant determined experimentally. The simplicity and effectiveness of this model made their way into the application of membrane filtration, such as the ultrafiltration of polyethylene glycol, in which all pore-blocking mechanisms performed similarly, with complete blocking (CB) having R
2 between 0.621 and 0.913, intermediate blocking (IB) having R
2 between 0.638 and 0.923, standard blocking (SB) having R
2 between 0.635 and 0.918, and cake formation (CF) having R
2 between 0.639 and 0.947 for crossflow velocities (CFVs) of 1-3 m/s and transmembrane pressures (TMPs) of 0.1-0.4 MPa [
2].
Applications in cross-flow ultrafiltration of effluent from a railway workshop yielded more consistent results for all four blocking mechanisms, with correlation coefficients between 0.75 and 0.88 for CB, between 0.88 and 0.92 for IB, between 0.83 and 0.91 for SB, and between 0.97 and 0.98 for CF for TMPs of 21, 35, and 48 kPa [
3]. Similar representative behavior is also found in the nanofiltration of polycyclic aromatic hydrocarbons in membranes NF270 and NF10, with CB having R
2 of 0.863 and 0.988, IB having 0.936 and 0.988, SB having 0.947 and 0.991, and CF having 0.908 and 0.957, respectively [
4].
Although the Hermia model can perform well with the original four values of
, there are applications where the permeate flux is discontinuous, such as the removal of glycerol from biodiesel at TMPs of 1 to 3 bar [
5,
6], or changes in pH in high-pressure wastewater nanofiltration [
7], in which these values of
cannot accommodate experimental data. Therefore, this model has been modified to better accommodate experimental results by increasing its complexity, either by keeping the same blocking mechanisms but changing the equations, such as in glycerin-water solutions, where simple changes in the original equations yielded R
2 between 0.6951 and 0.8611 for complete blocking, between 0.8489 and 0.9622 for intermediate blocking, between 0.8189 and 0.9315 for standard blocking, and between 0.7896 and 0.9468 for cake formation [
8].
Other modifications can be applied by using the concept of flow resistance, as in the microfiltration of oil-in-water emulsions, in which a modified Hermia model is used in conjunction with the first and second derivatives of the flow resistance. This setup yields a system of ODEs that results in the behavior of flux,
[
9]. Still, both these modifications use the original Hermia model as the base. Similar approaches have been used to model fouling in micro- and ultrafiltration membranes for treating limed and partially clarified sugar cane juice, such as the use of cake filtration (CFM) and combinations of external and progressive internal fouling models (CEPIFM). In the case of treating limed and partially clarified sugar cane juice, these models can perform well, with R
2 between 0.9939 and 0.9992 for CFM, and 0.9718 and 0.9883 for CEPIFM [
10].
Although the use of more complex models, such as CFM and CEPIFM does indeed improve the predictability of and the accumulated volume of permeate, it comes at the cost of simplicity. Therefore, this paper aims to mathematically prove a more general version of the Hermia model, as well as make fitting experimental data simpler and more effective without having to use complicated equations. We have also aimed to use the extended Hermia model in different examples and compare its performance to the original four pore-blocking mechanisms (Eq. 1-4).
3. Results
Taking into account Eq. 1-4, it is possible to observe that, apart from
,
seems to follow a pattern, such that, if the reduced permeate flux (
) is isolated in Eq 2-4:
| Intermediate blocking () |
|
Eq. 13 |
| Standard blocking ( |
|
Eq. 14 |
| Cake formation() |
|
Eq. 15 |
Since
is a real number and
is a constant, Eq. 12-14 can be rewritten as one equation (Eq. 16) with a variable exponent
, where
.
In this context, the pore-blocking mechanisms would be given by different values of
, such that
is intermediate blocking,
is standard blocking, and
is cake formation. Furthermore, it is possible to establish a relationship between
and
, such that
. For
(or
), the reduced permeate flux is simply given by Eq. 17.
We have wondered if other values of can be used in Eq. 16 to better represent experimental data, expanding the original model into a sort of extended Hermia model (EHM). Therefore, we have performed the model fitting for all four original pore-blocking mechanisms and the EHM in Examples 1, 2, 3, 4, 5, and 6 to have a better understanding of how these mechanisms change in different contexts. In these Examples, we have obtained consistently better performance than the four original pore-blocking mechanisms. Thus, to justify the use of the EHM, we have also used Eq. 5-11 and proven Theorems 1, 2, and 3. Their proofs can be found in Appendices A, B, and C, respectively.
Theorem 1.
The original Hermia model can be extended to accommodate new values of P. If both the fluid and the permeate have similar densities, then the flux can be expressed by Eq. 18 for any . If , then then the flux can be expressed by Eq. 19.
A measure of how fast declines over time can be given by applying both Eq. 18 and Eq. 19 and calculating the amount of time needed for the reduced permeate flux to drop by half . We will refer to this quantity as the EHM half-life (Eq. 20).
Therefore, for as given , there is a pth-degree blocking mechanism. This means intermediate blocking is a 1st-degree blocking mechanism, that cake formation is a 2nd-degree blocking mechanism, that, and so on.
Theorem 2.
If the EHM has been correctly fitted to experimental data and represents the dataset well (such as with a low RMSE or with a high R2), then the fouling layer’s thickness can also be fitted to the profile given by Eq. 21.
Theorem 3.
If the EHM has been correctly fitted to experimental data and represents the dataset well (such as with a low RMSE or with a high R2), then the accumulated permeate volume can be calculated using Eq. 22.
Example 1. Model fitting for ultrafiltration membrane used in different wastewater pretreatment conditions
In a paper by Jung and Son, a pretreatment of organic matter coagulation and MIEX® was evaluated on a bench-scale filtration apparatus. This work investigated many different pretreatment conditions and their impact on micro- and ultrafiltration in hydrophilic (HPI) and hydrophobic (HPO) membranes. While keeping TMP at 1 bar for microfiltration and 2 bar for ultrafiltration, both coagulant and MIEX® were added to the wastewater and the filtration was carried out [
15].
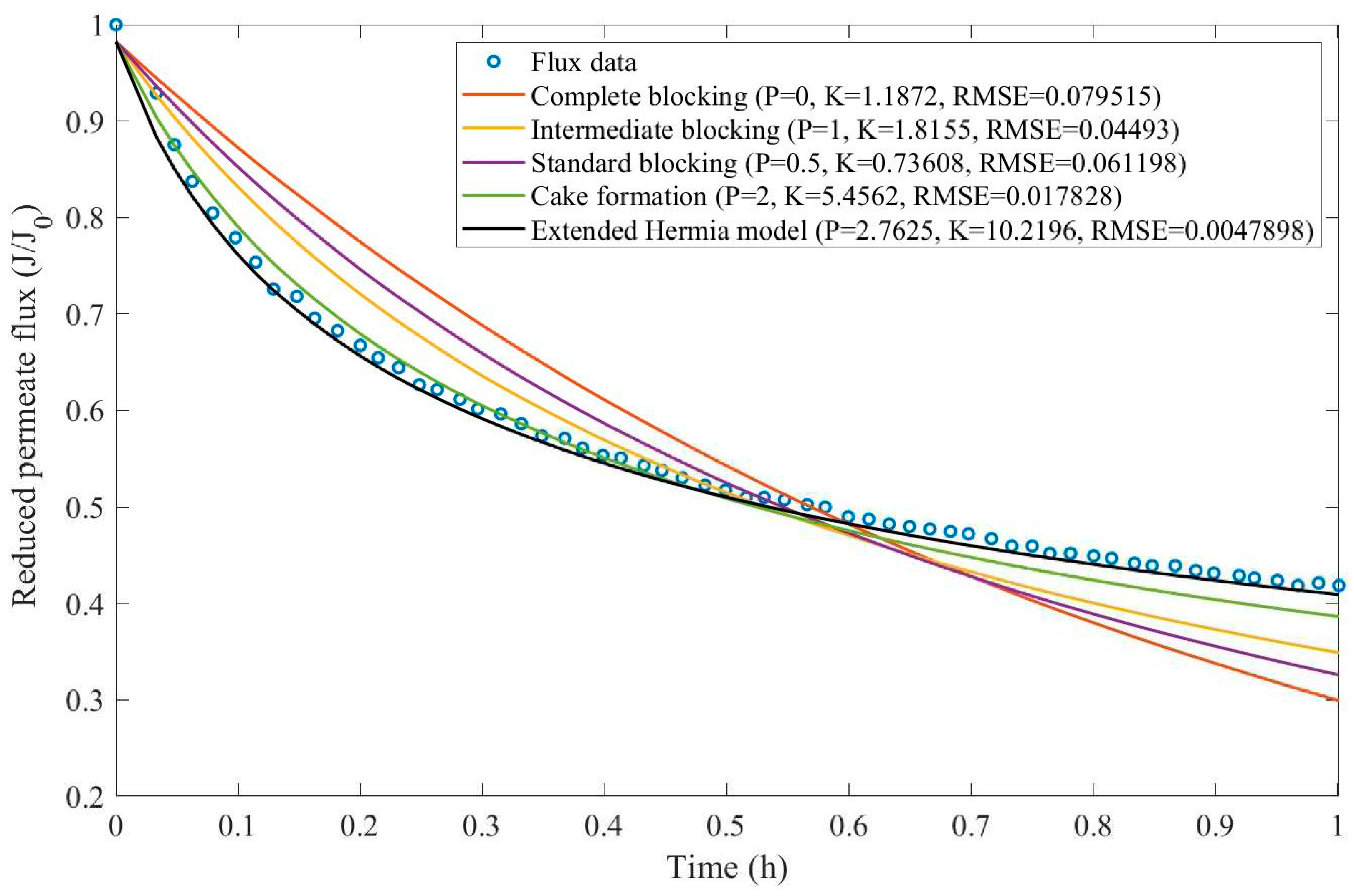
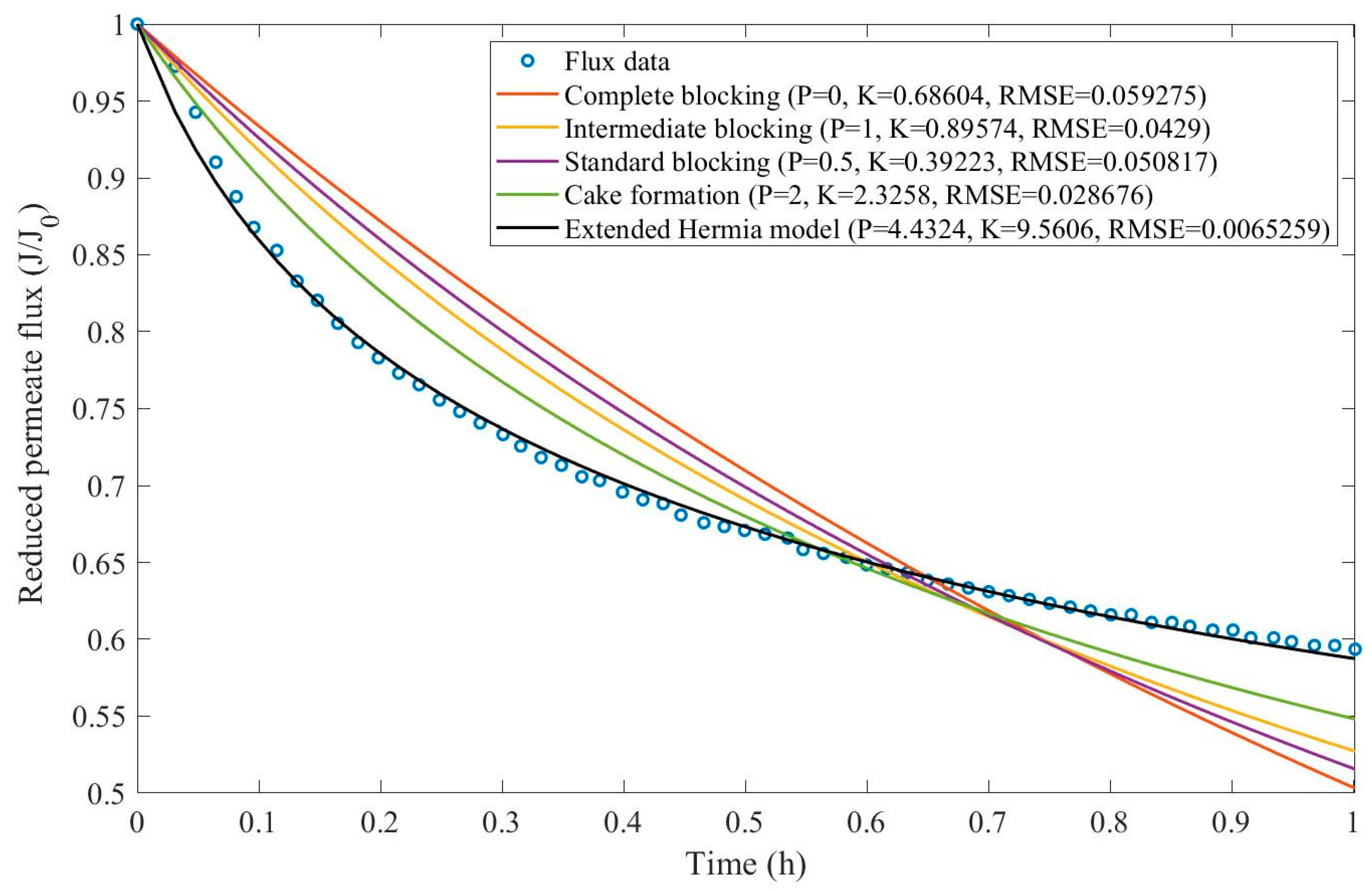
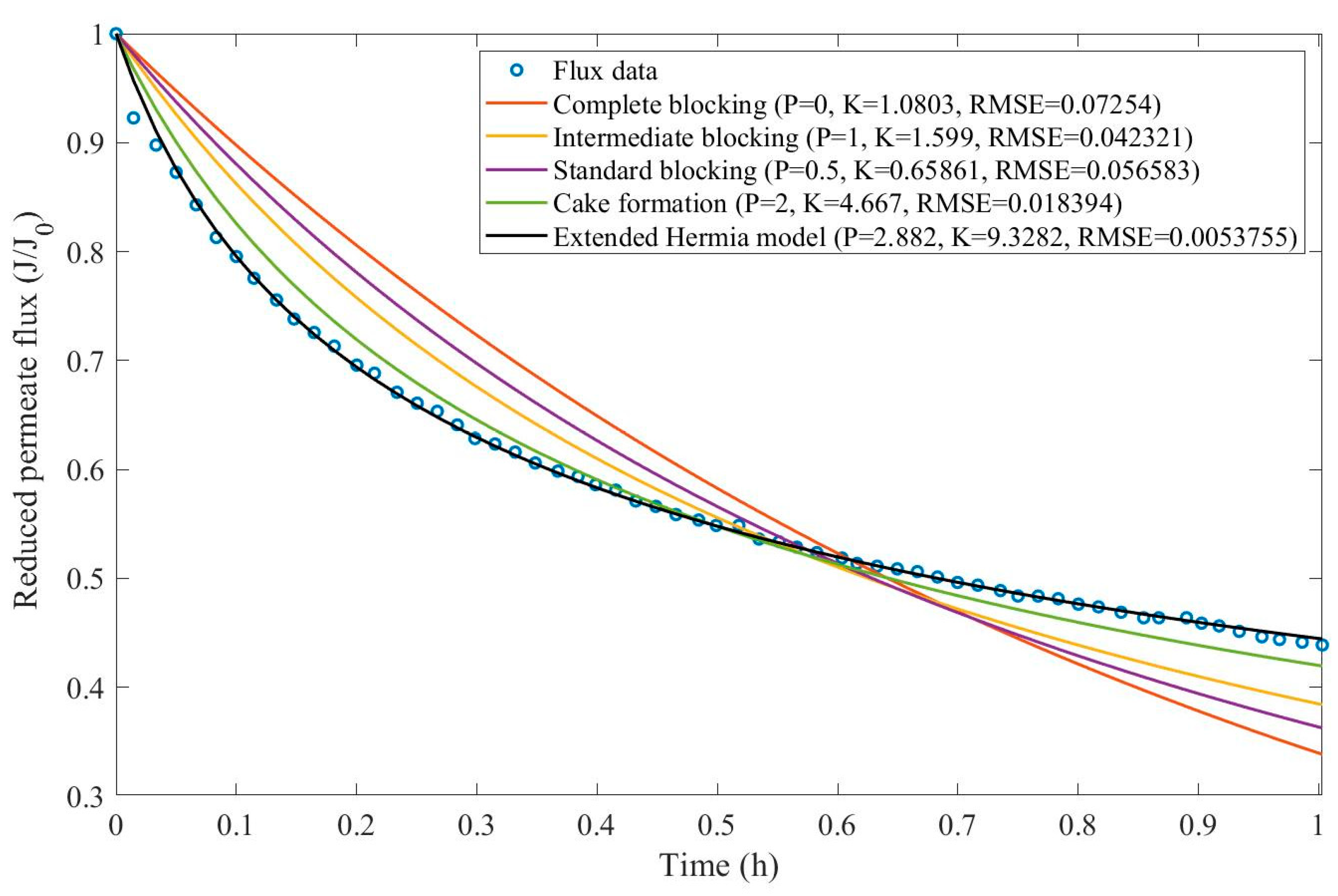
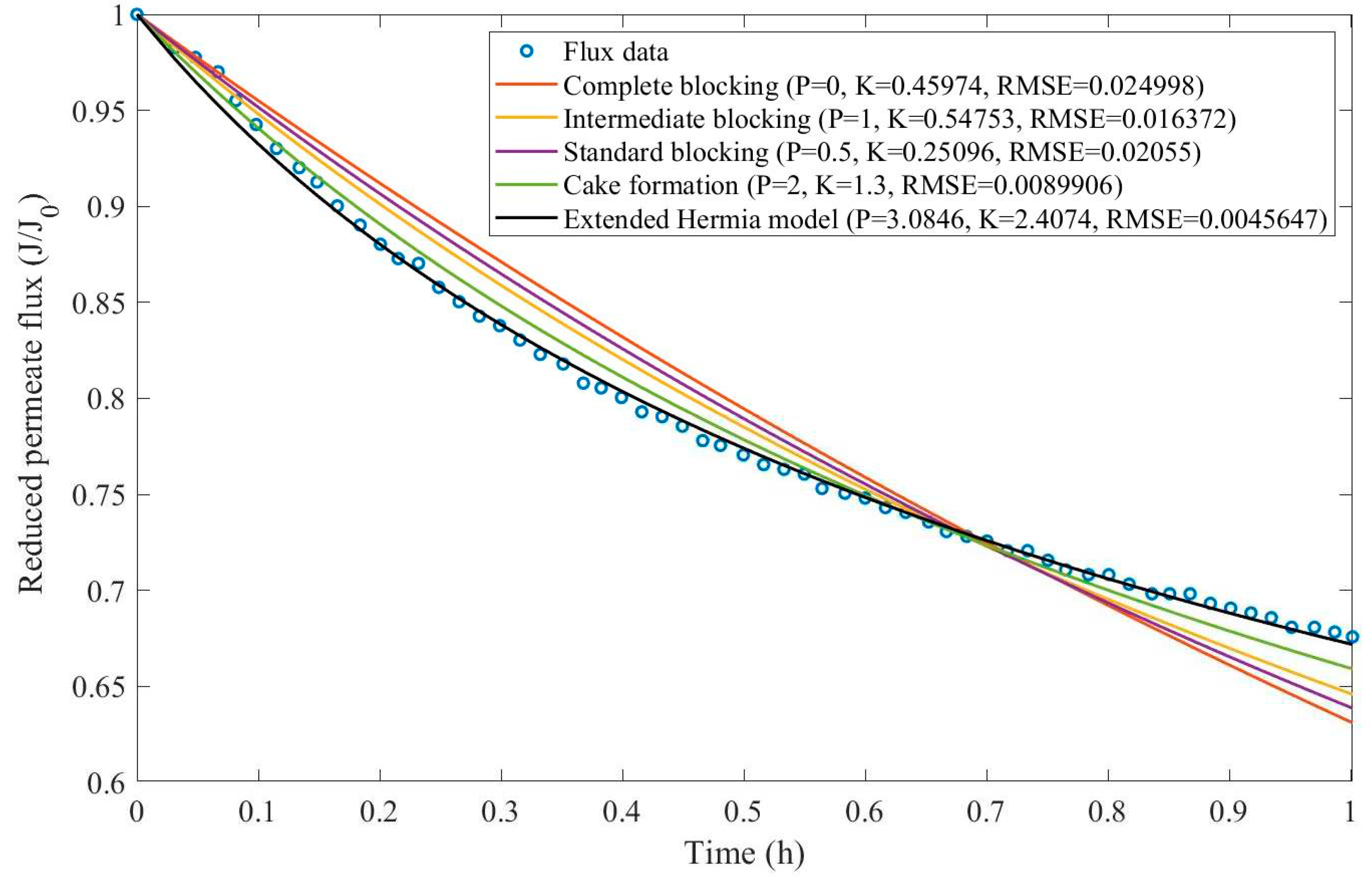
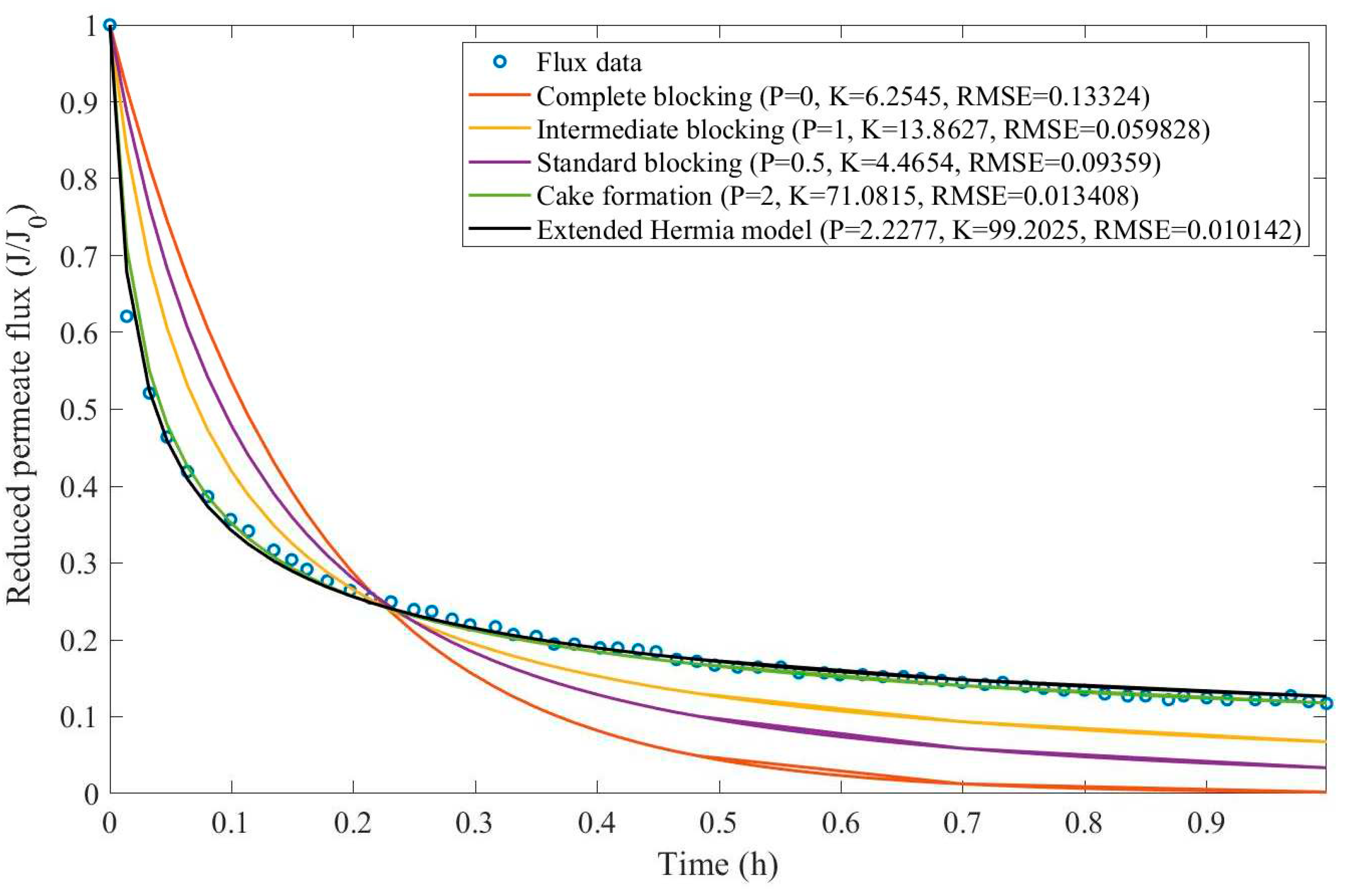
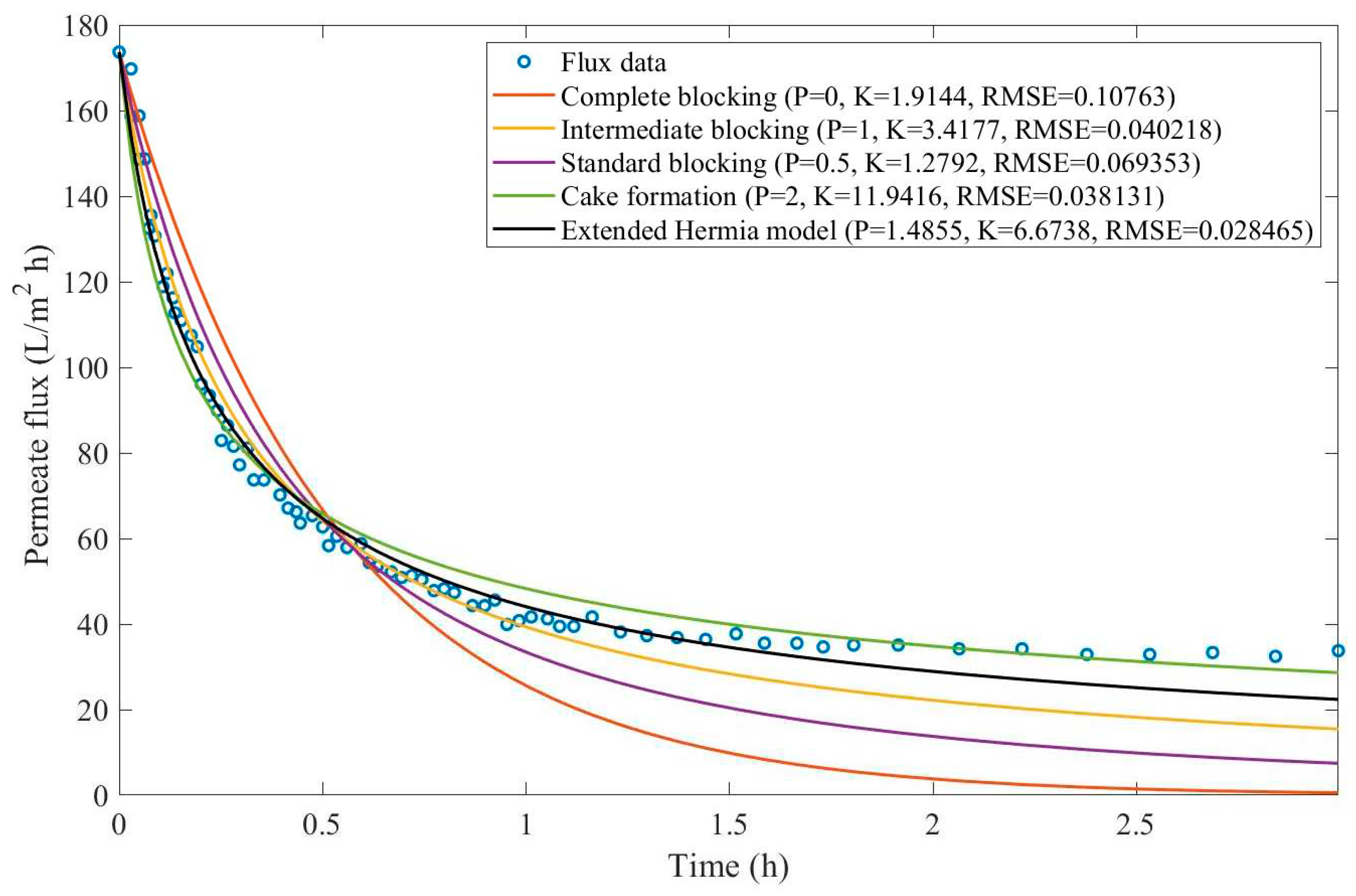
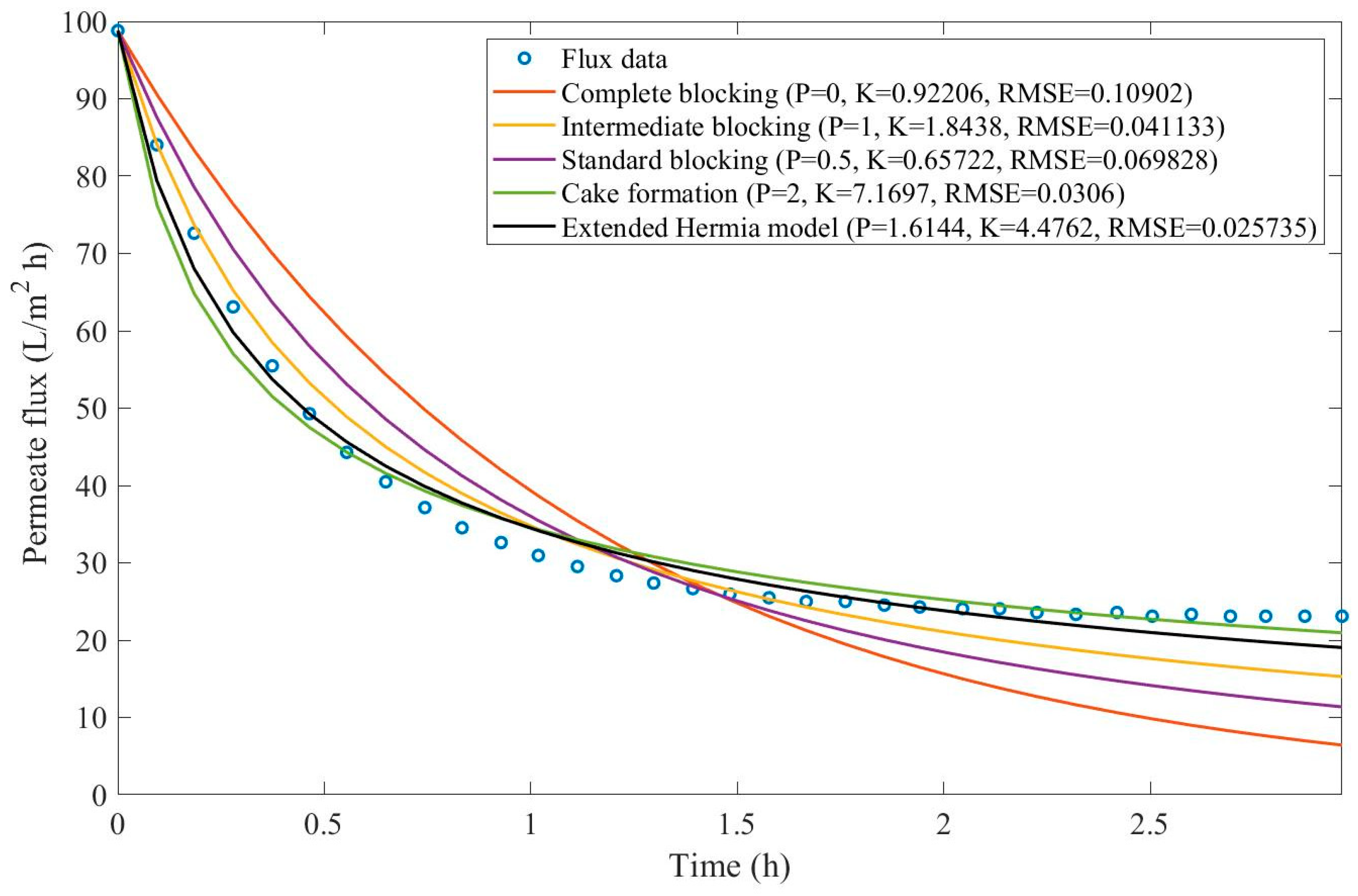
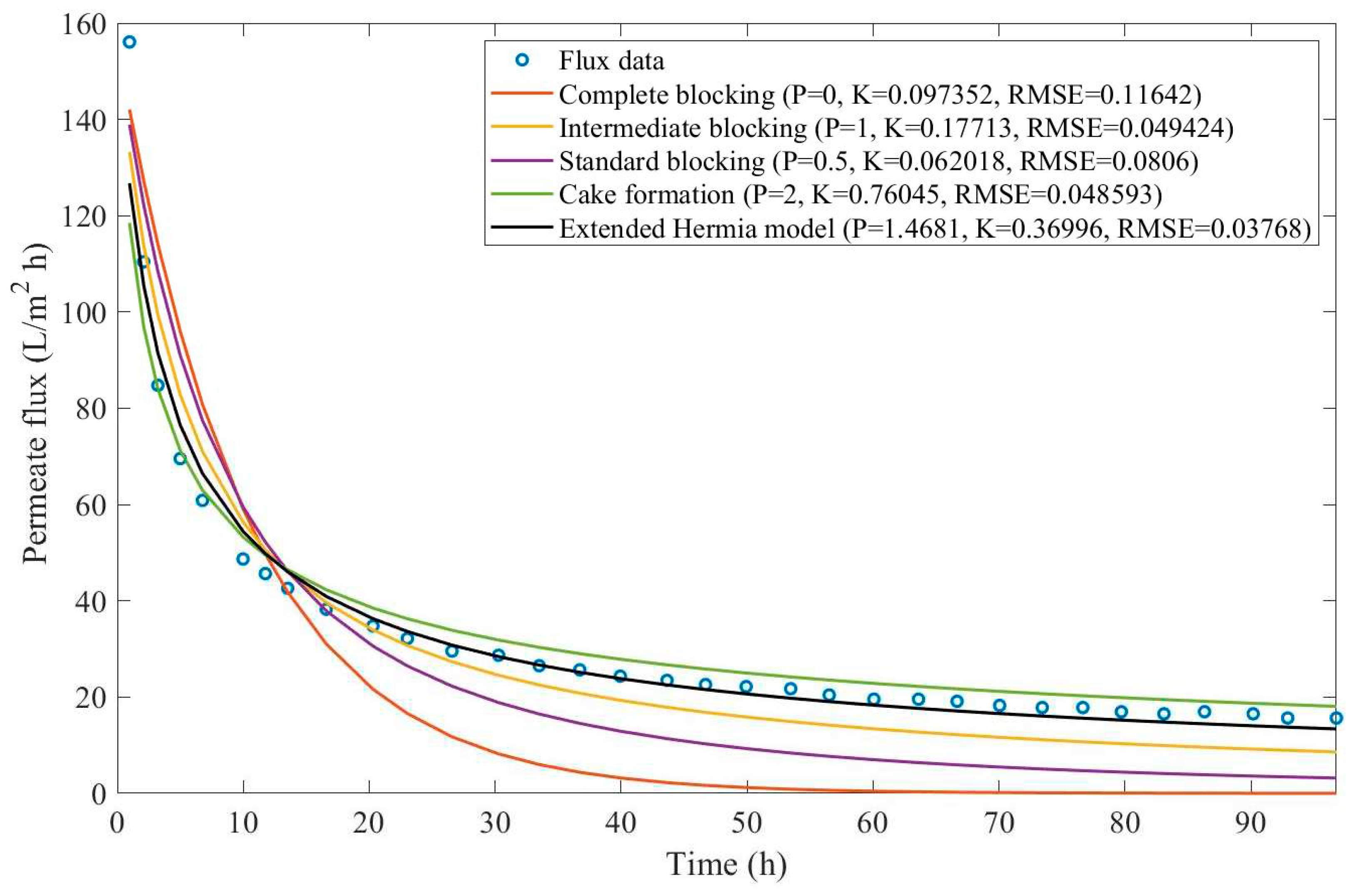
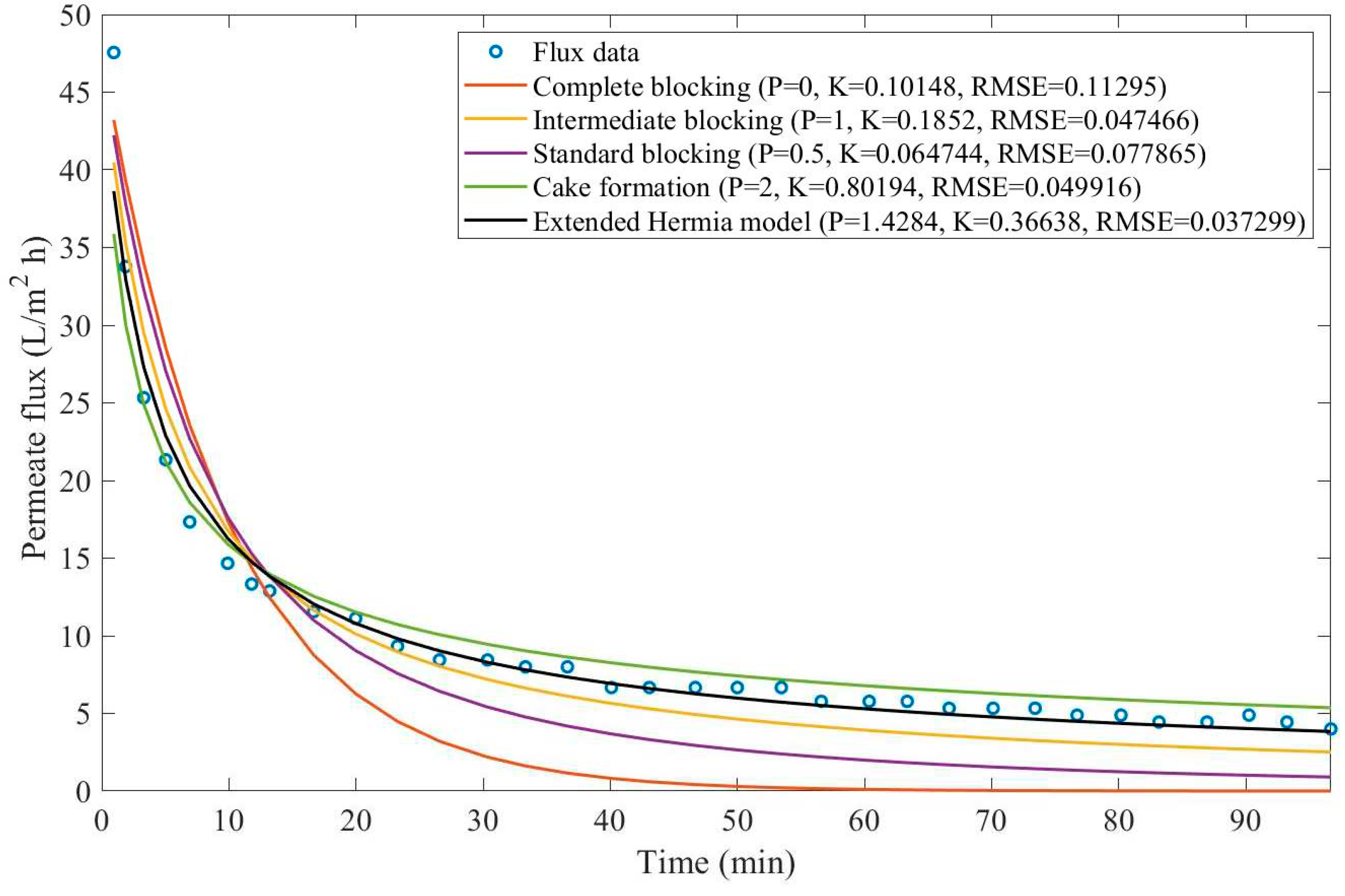
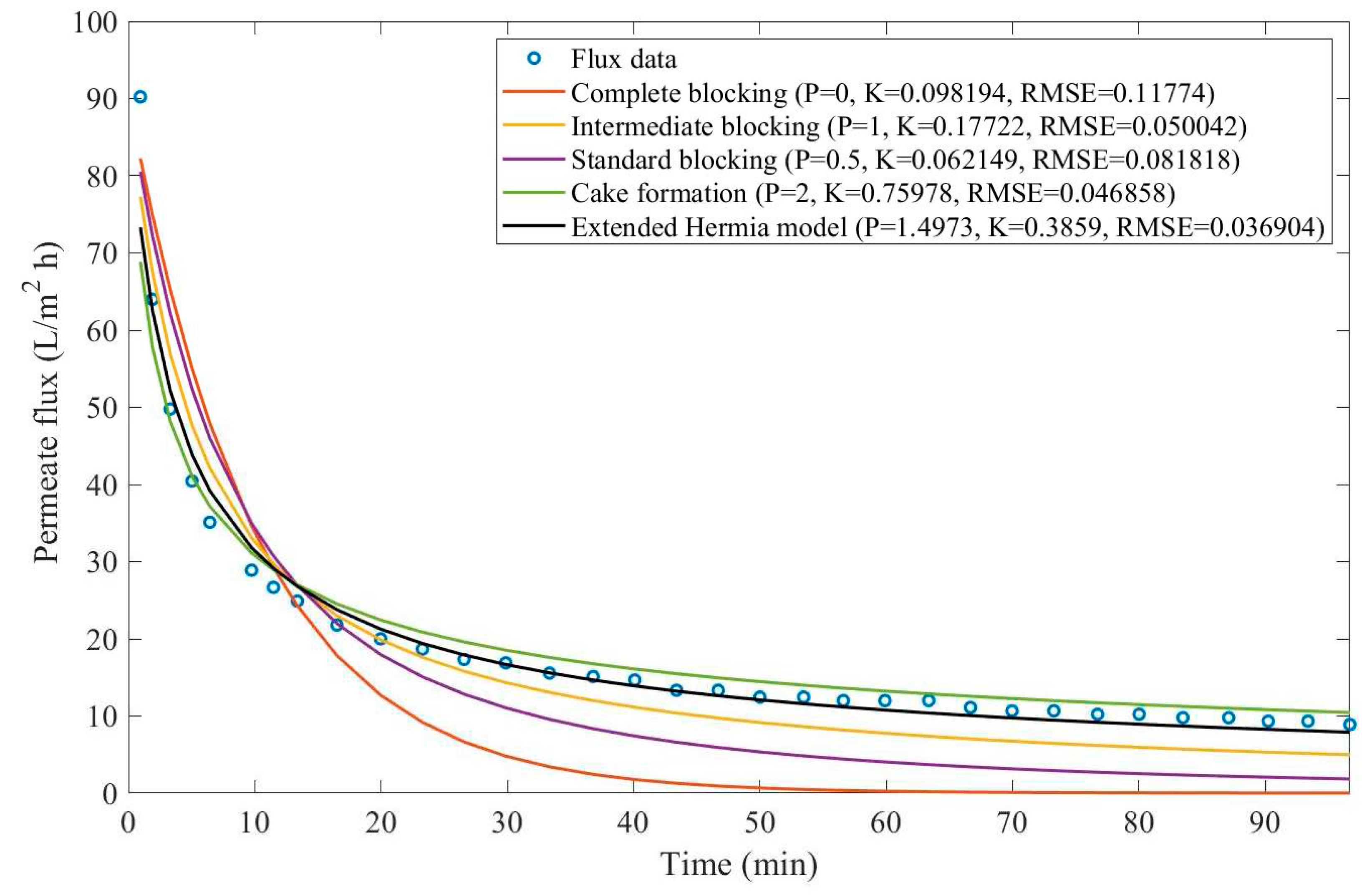
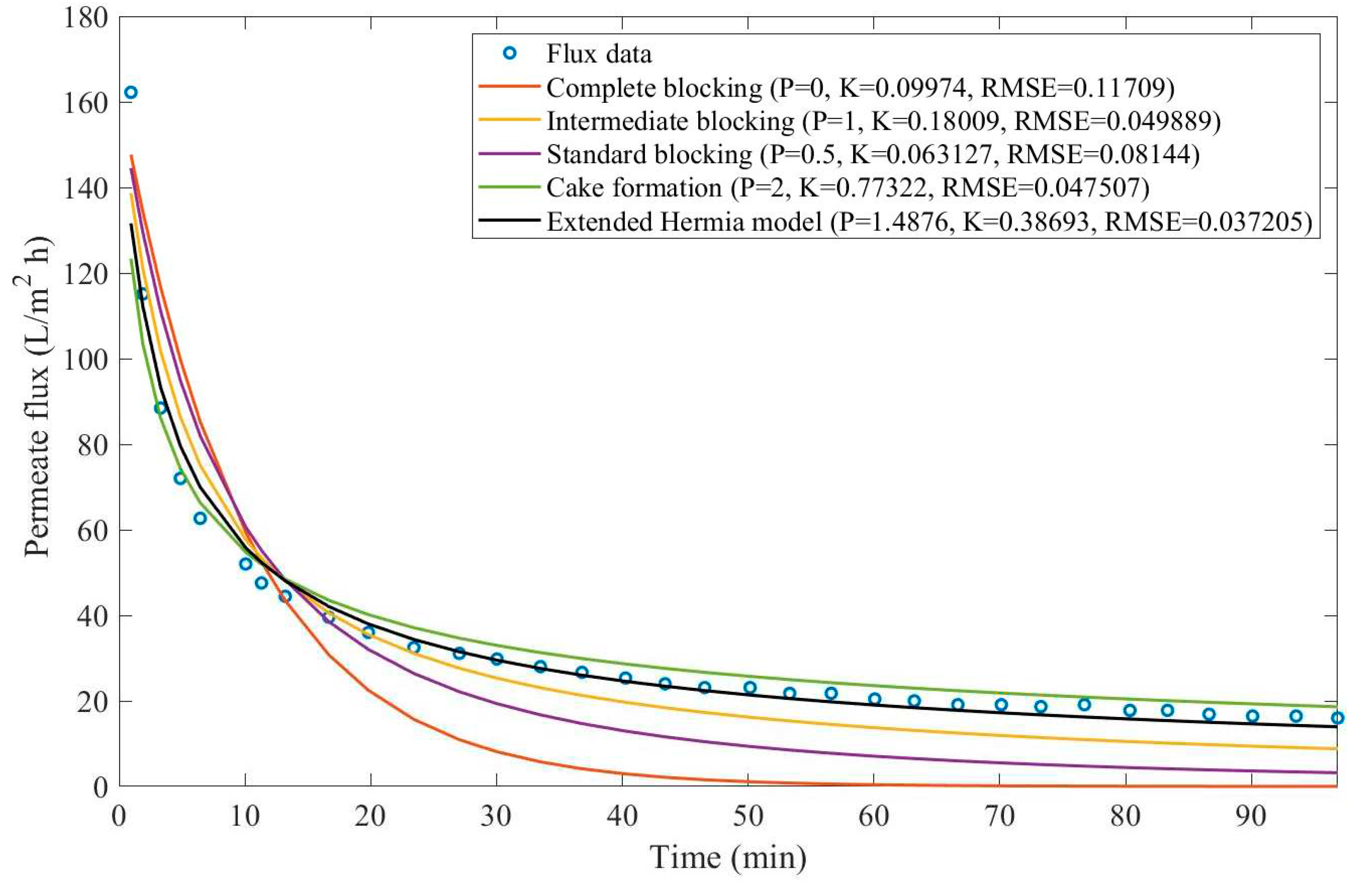
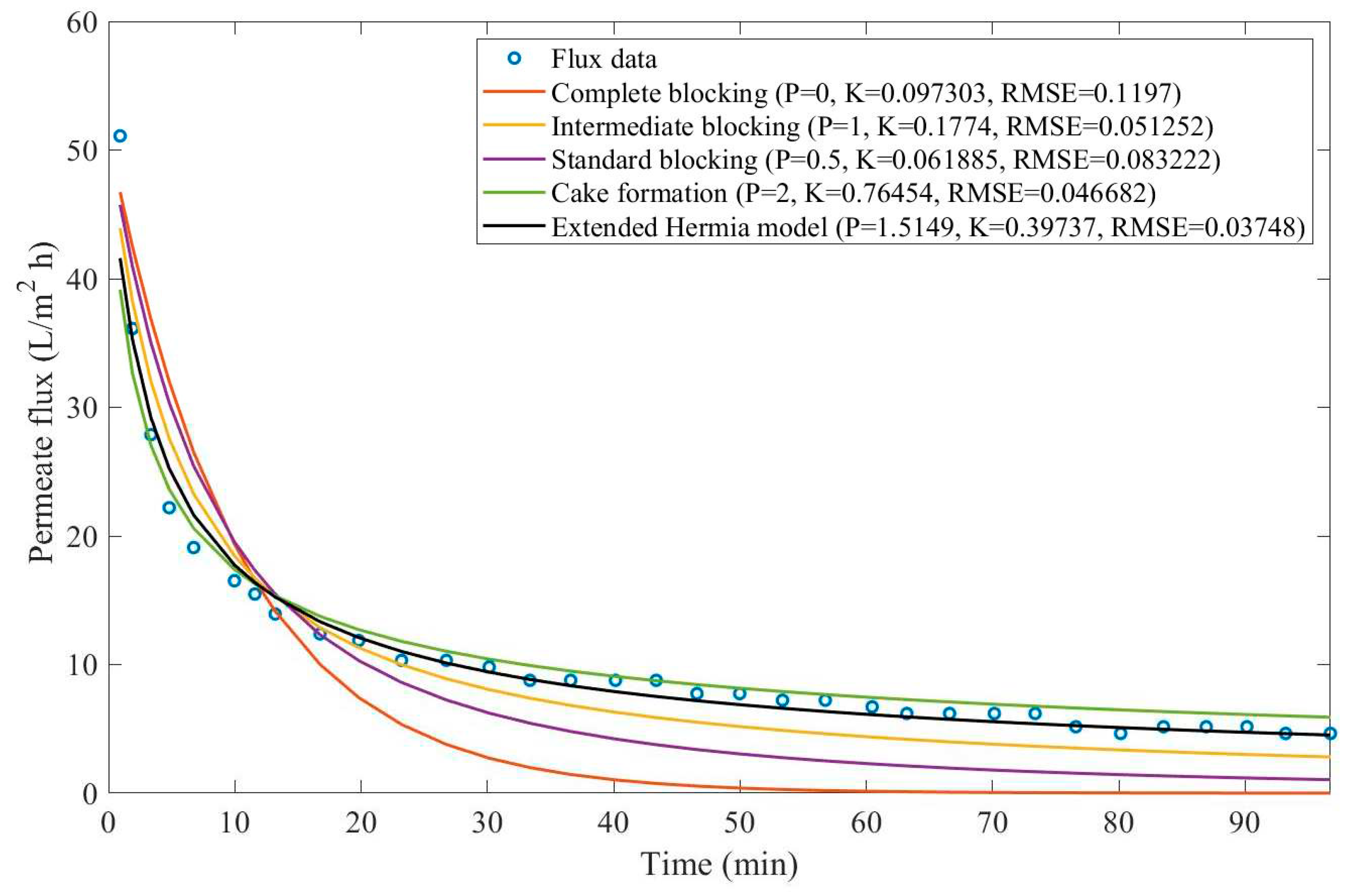
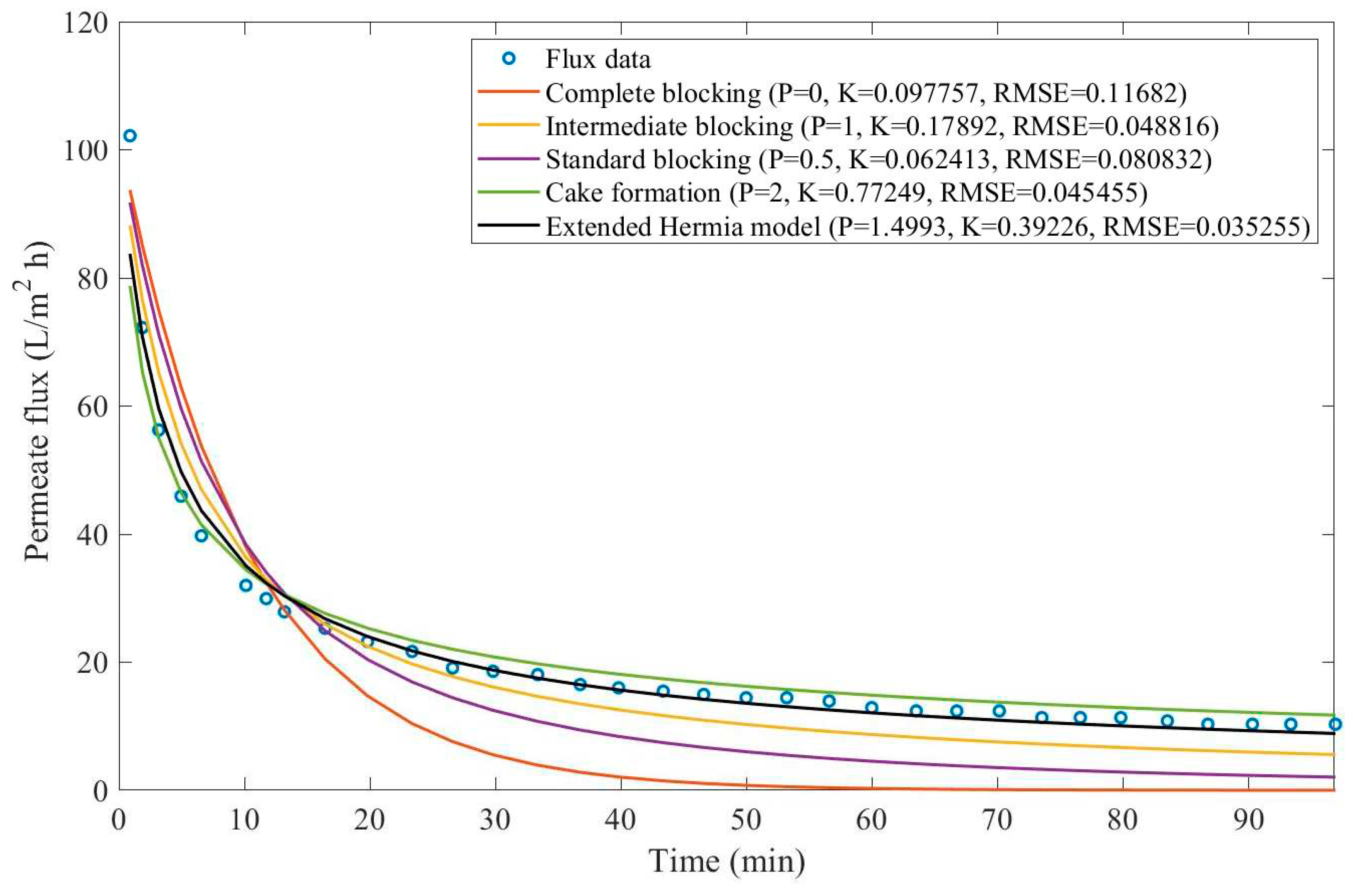
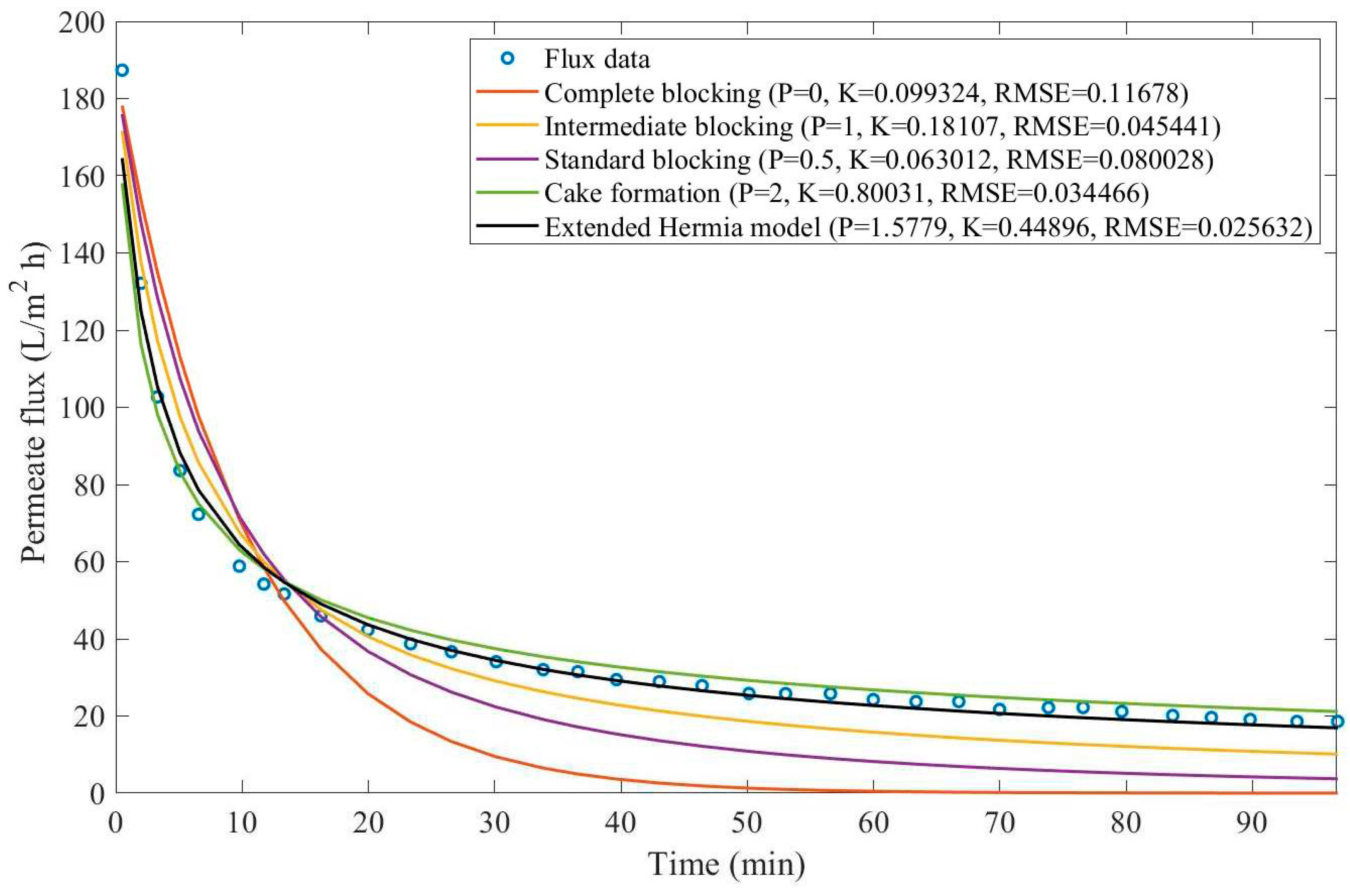
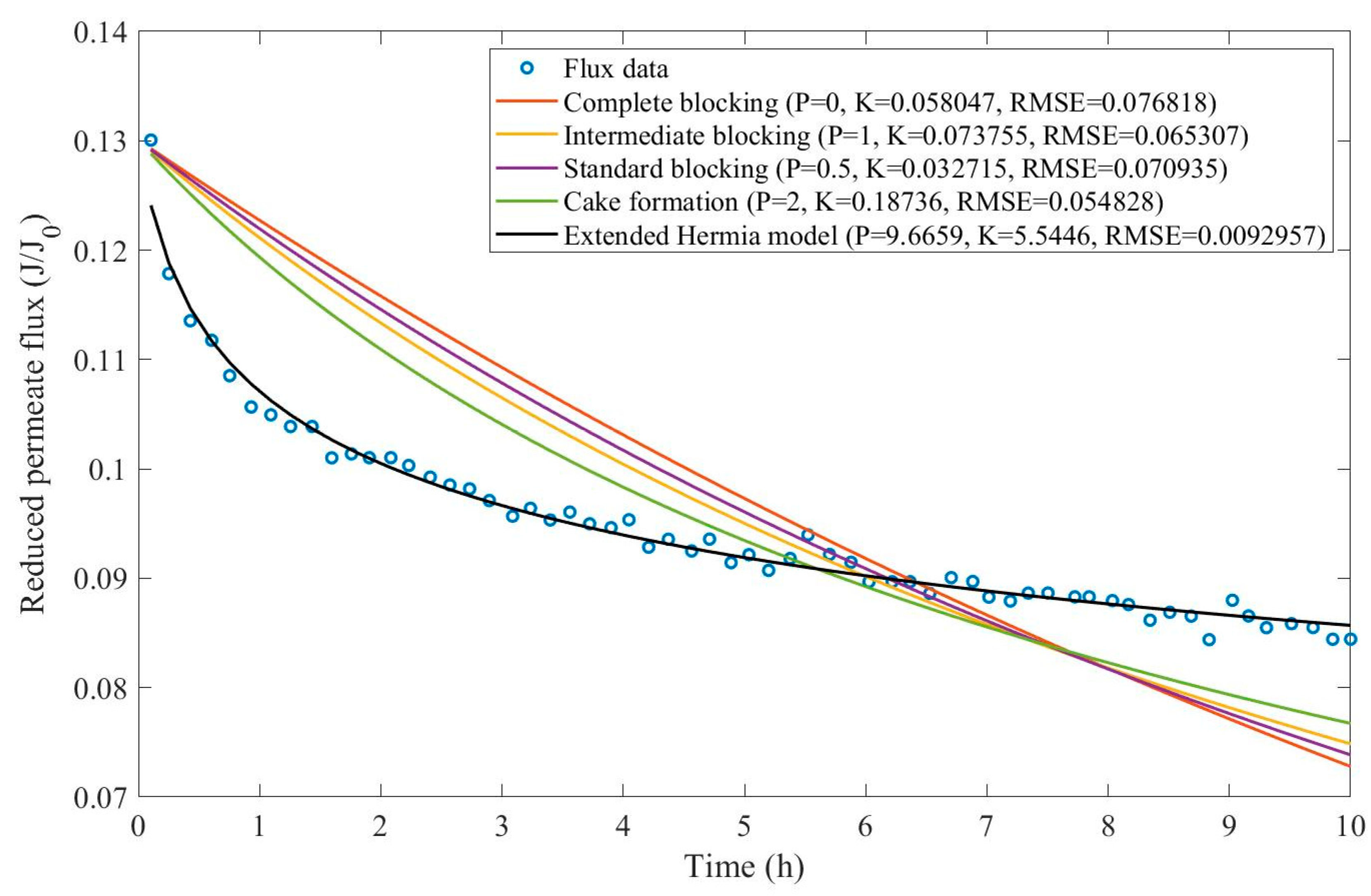
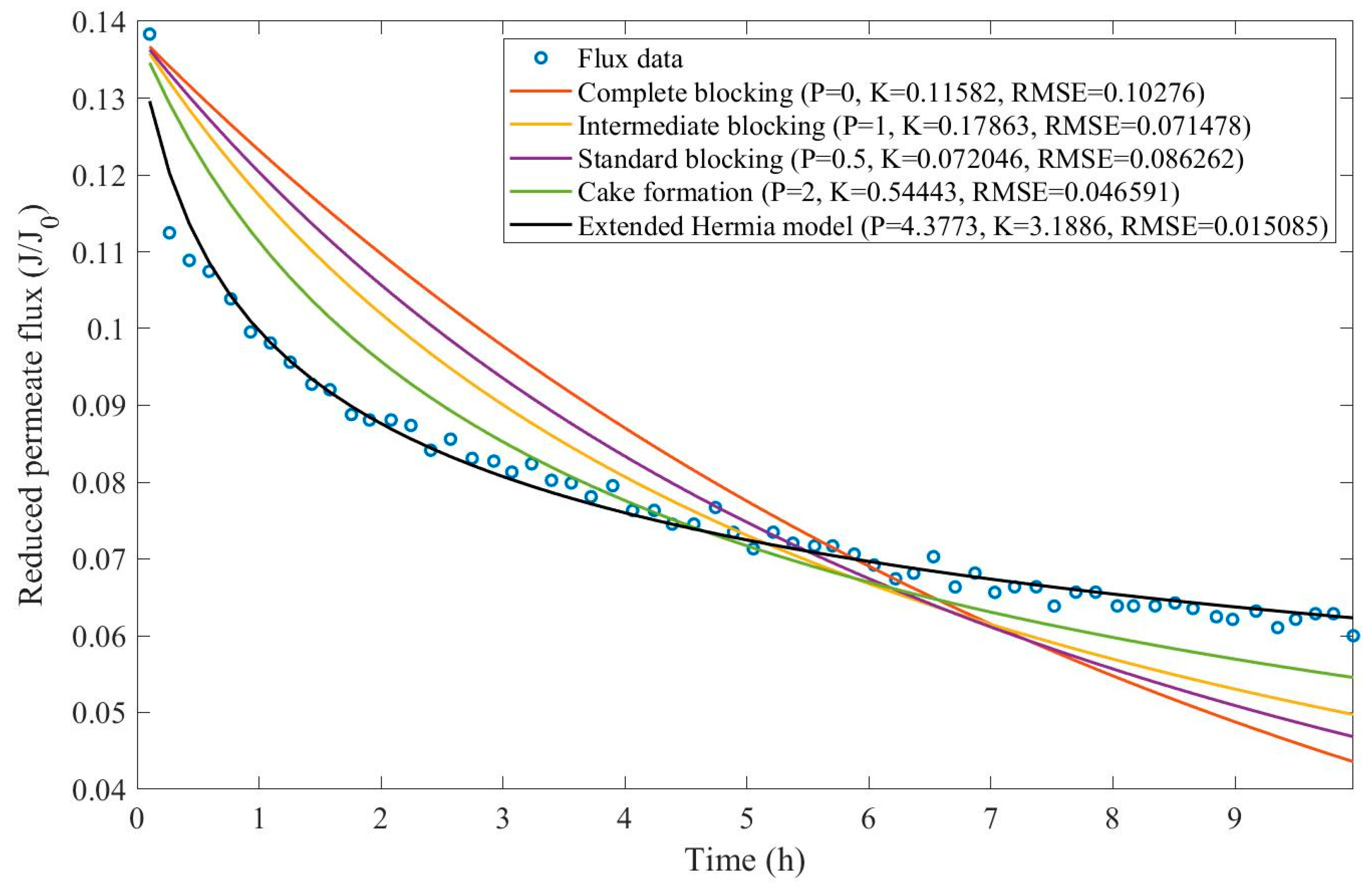
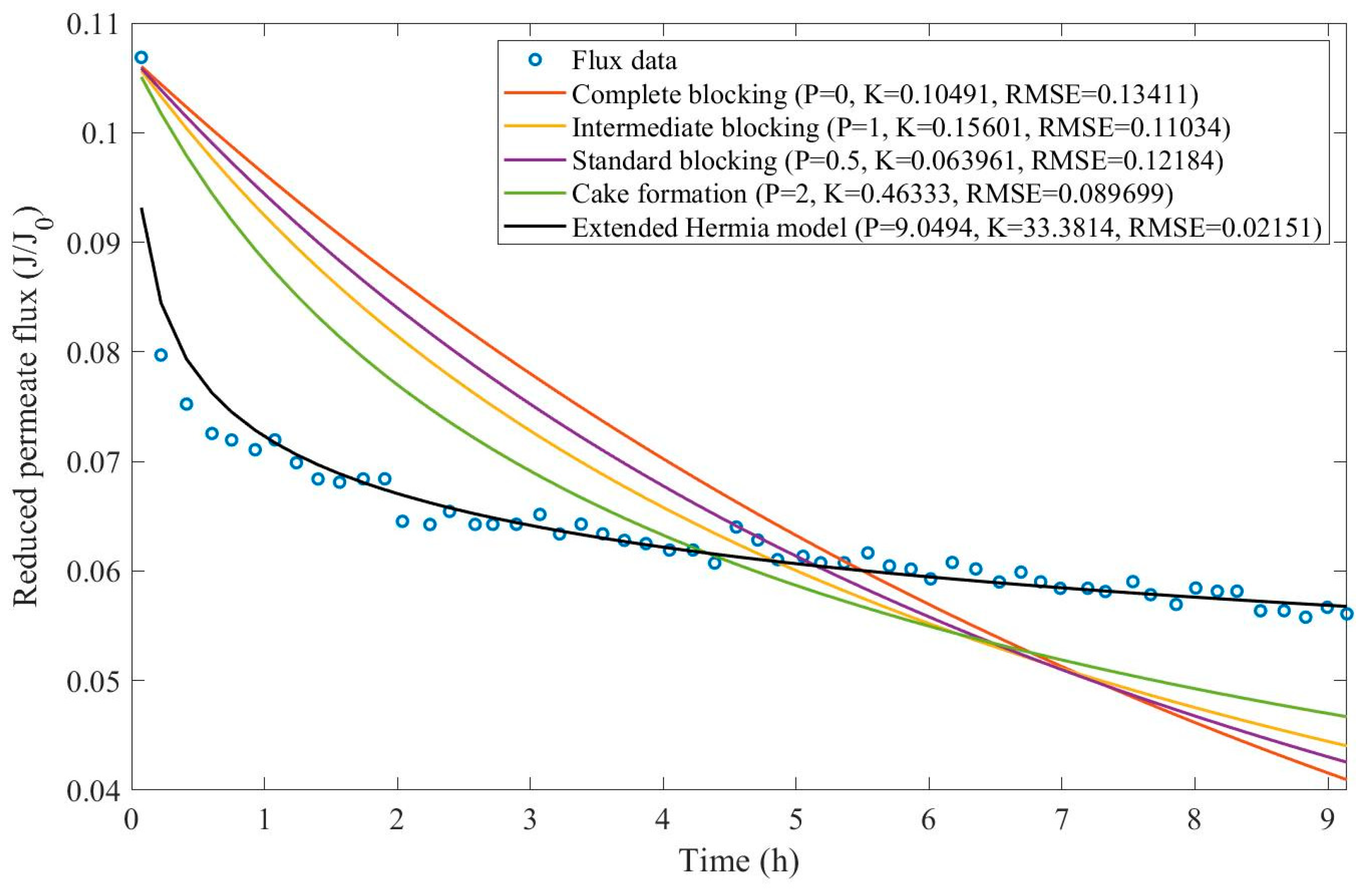
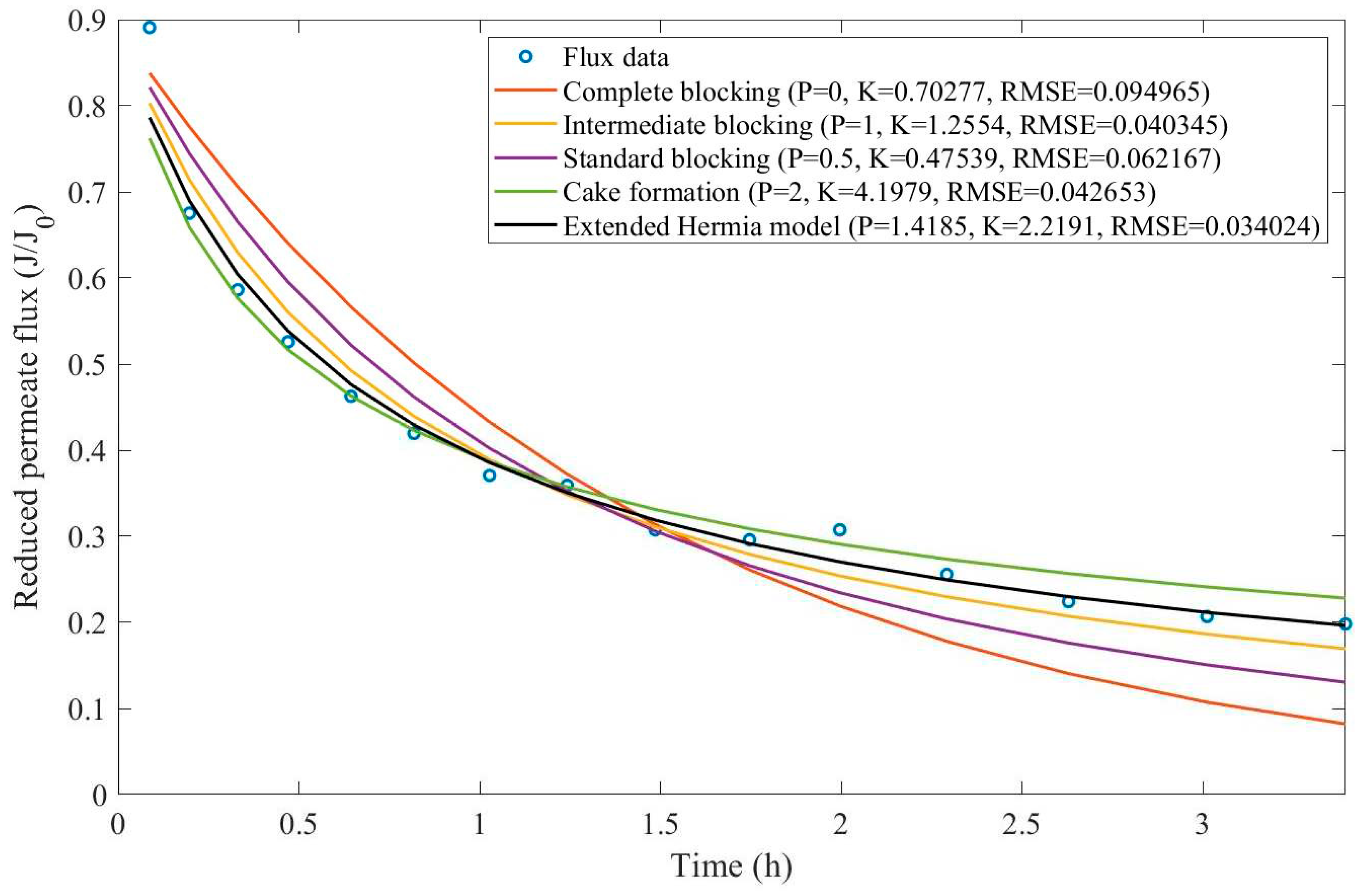
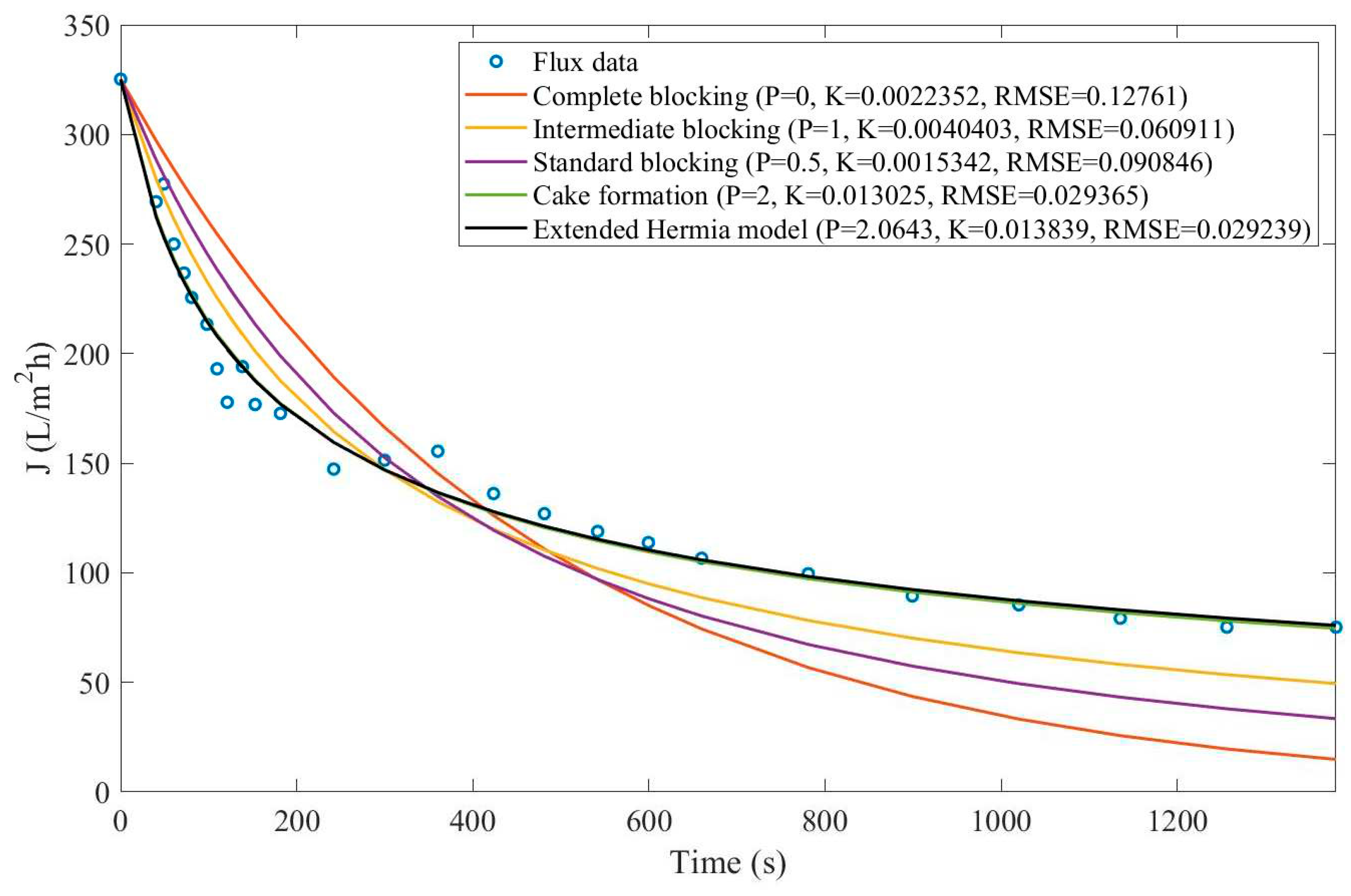
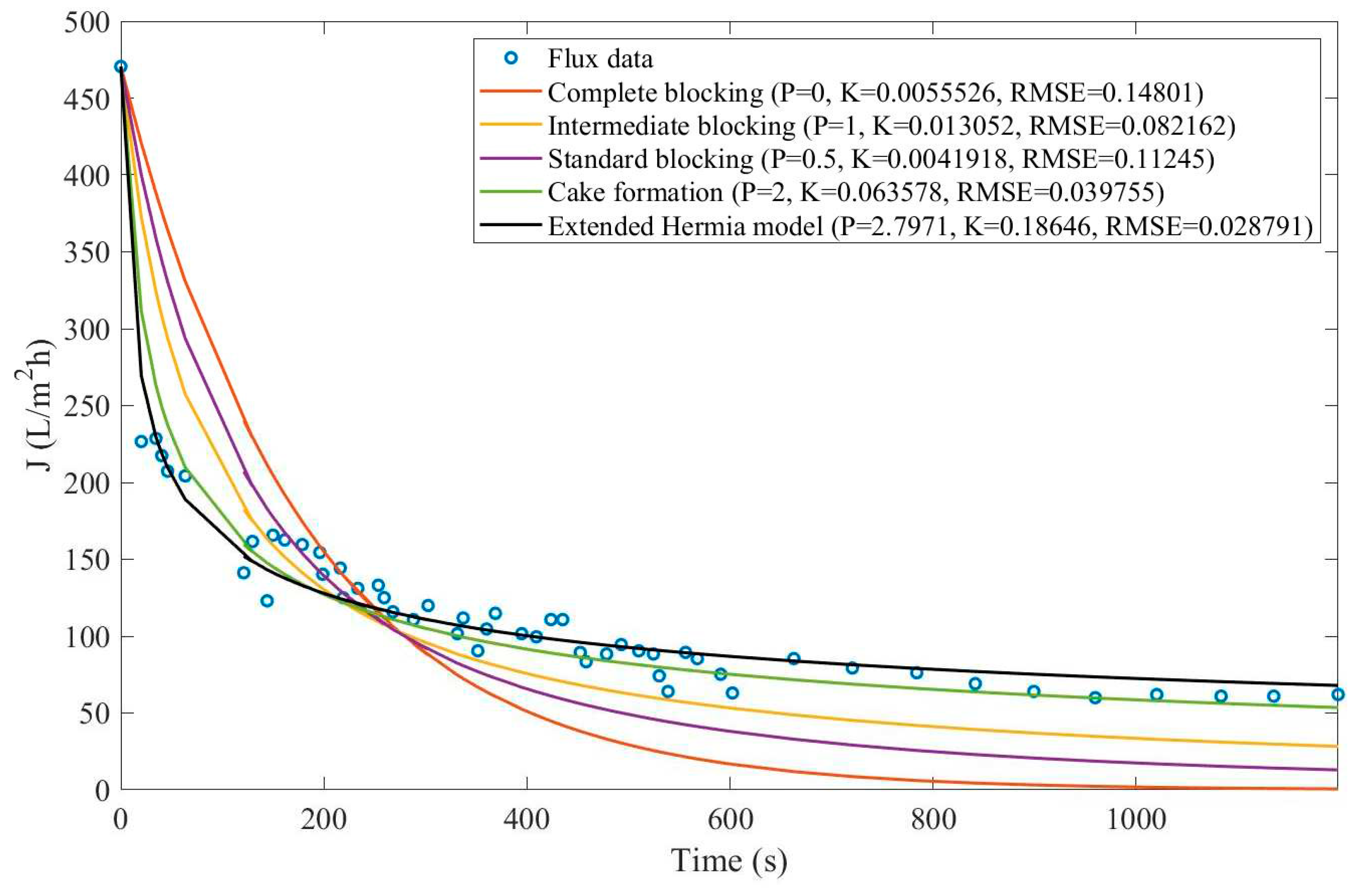
In this example, we have isolated the data obtained from ultrafiltration for both HPI and HPO membranes with and without the addition of coagulant 140 mg/L and MIEX® 12 mL/L. We have performed the model fitting for all four pore-blocking mechanisms and the extended Hermia model by minimizing the root mean square error (RMSE). These regressions can be found in
Appendix D (Figure D 1-Figure D 8),
Table 1, and
Table 2.
Based on the regressions obtained, we have noticed that the extended Hermia model has a better performance when comparing the four blocking mechanisms (RMSE≤0.01), followed by the cake formation mechanism. Although the EHM provides better estimates for flux, cases 1, 2, 3, 4, 5, 6, and 8 have , which can be physically interpreted as a new blocking mechanism.
For cases 5 and 7 we have obtained values of that are relatively close to the cake formation mechanism which implies that this type of blocking can happen to a certain degree. As an example, case 7 shows that , therefore, we can physically interpret this as a mixture of both cake formation and intermediate blocking. As for cases 3, 5, and 8, the same principle can be applied, therefore, these cases indicate a mixture of cake formation and a 3rd-degree blocking mechanism. Comparing both HPI and HPO membranes with no additions, the EHM predicts that the HPI membrane has a half-life of 0.57 h, meanwhile the HPO membrane has a half-life of only 0.04 h. This indicates that for this example fouling greatly affects HPO membranes when compared to HPI membranes. We have also noticed that the addition of coagulant and MIEX® increased the half-life for both HPI and HPO membranes.
This effect can be explained by the changes in the pore-blocking mechanism since the values of
change with the addition of coagulant and MIEX®. With no additives the mechanism tends to cake formation (
for HPI and
for HPO) but the addition of coagulant shifts to a 4
th-degree blocking mechanism for HPI and a 3
rd-degree blocking mechanism for HPO (
for HPI and
for HPO). The addition of MIEX® changes the blocking mechanisms slightly (
for HPI and
for HPO). As a result, we can infer that the most significant change to the pretreatment is the addition of the coagulant, which increases EMH half-life considerably by changing the pore-blocking mechanism. Therefore, given the results presented in
Table 1, both additives used with the HPI membrane give a considerable increase in EMH half-life, which indicates that this is a better solution for fouling reduction in Example 1.
Example 2. Model fitting for microfiltration with ceramic membranes used in corn syrup clarification
In a paper by Almandoz and coauthors, 3 different ceramic membranes (CM08, CM05, and CM01) were evaluated at different CFVs and TMPs considering the removal of undesired oil, protein, and other non-starch components. The main difference between the ceramic membranes is their structure, mainly represented by properties such as mean pore radius obtained through volume mercury penetration (
), hydraulic permeability (
) and porosity (
). Microfiltration was carried out at 0.5 m/s and 50 kPa for all three membranes and CM05 was chosen for the following experiments due to better performance such as lower turbidity, lower concentrations of insoluble residues, and total proteins [
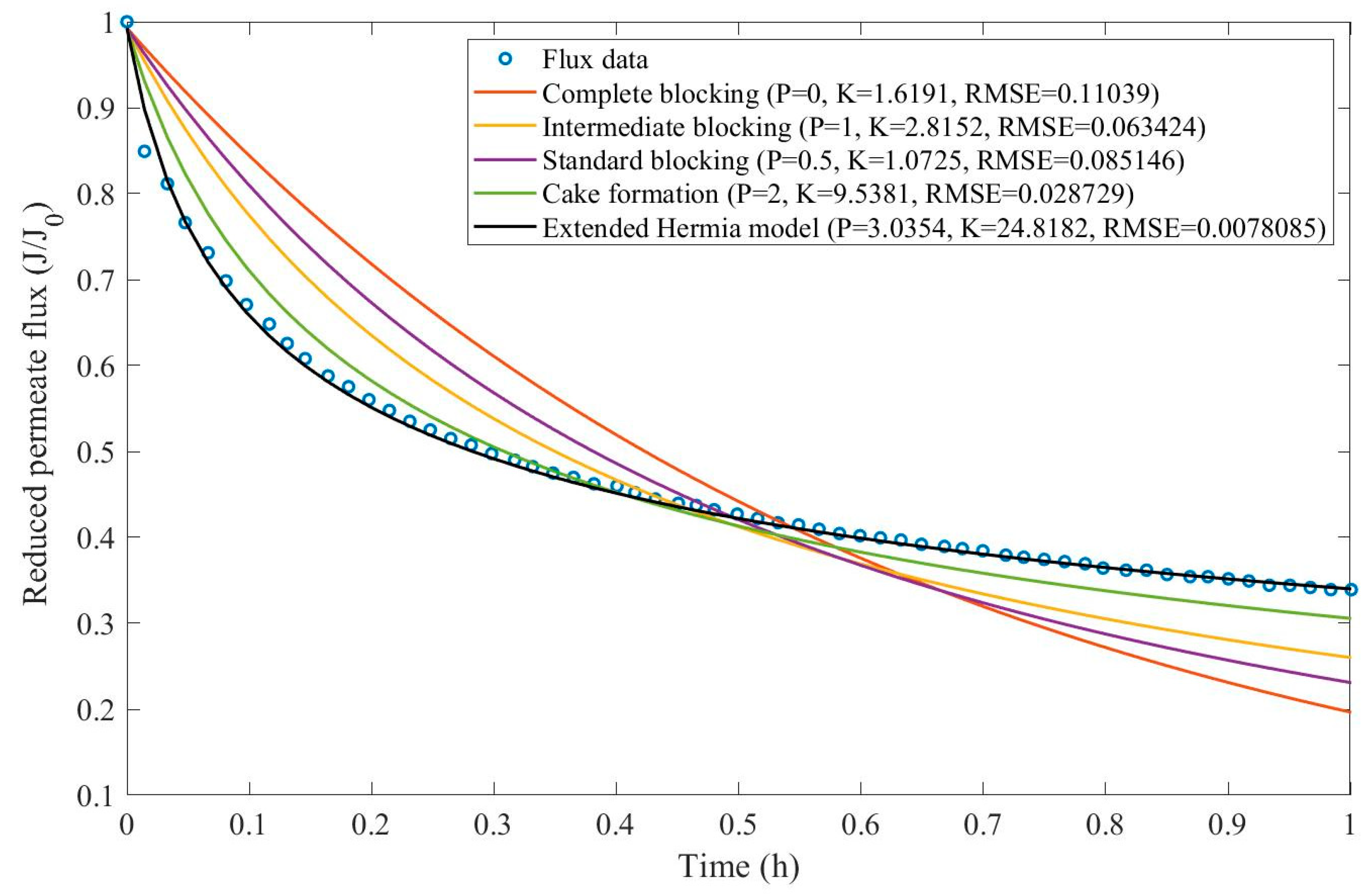
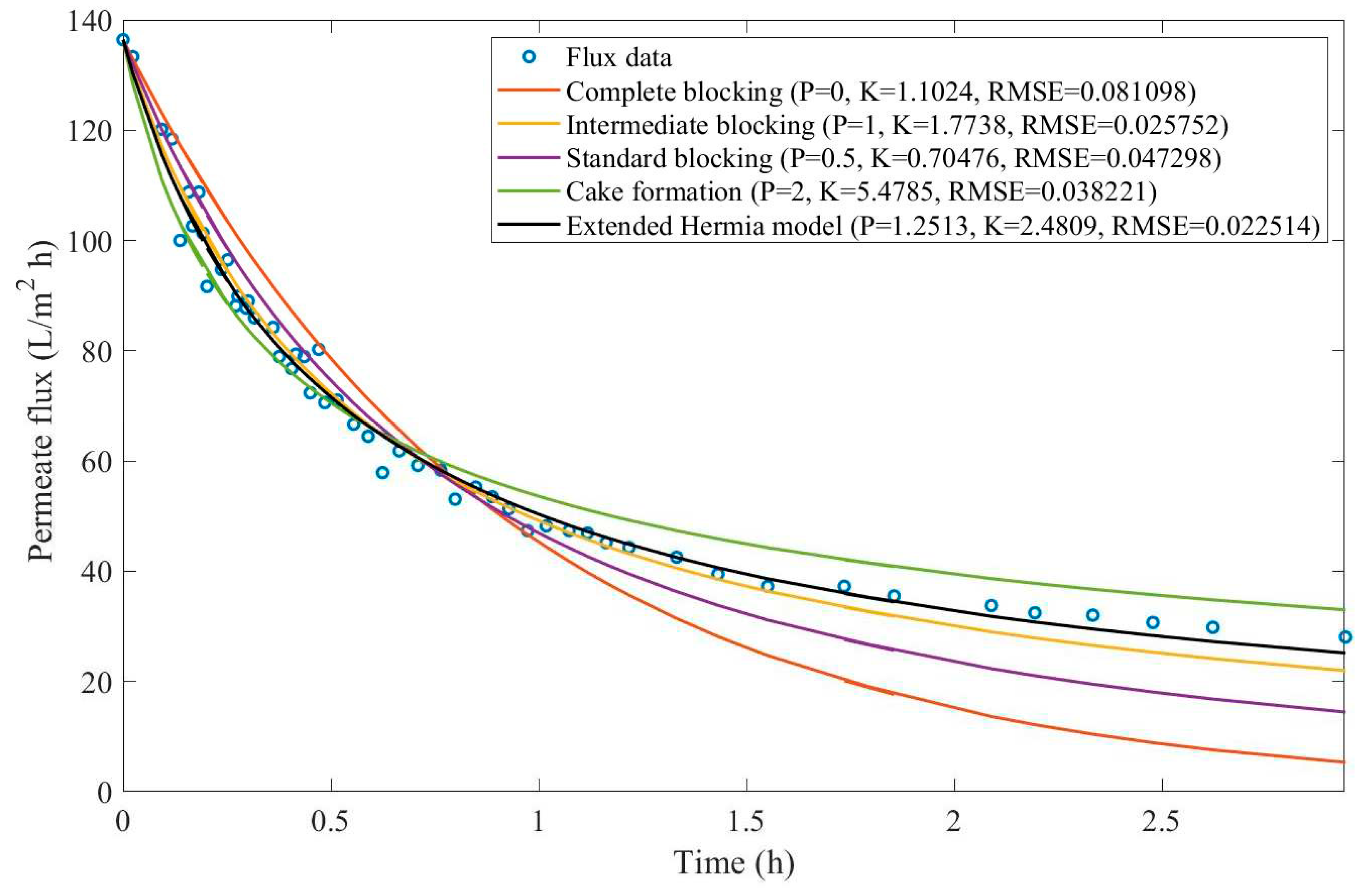
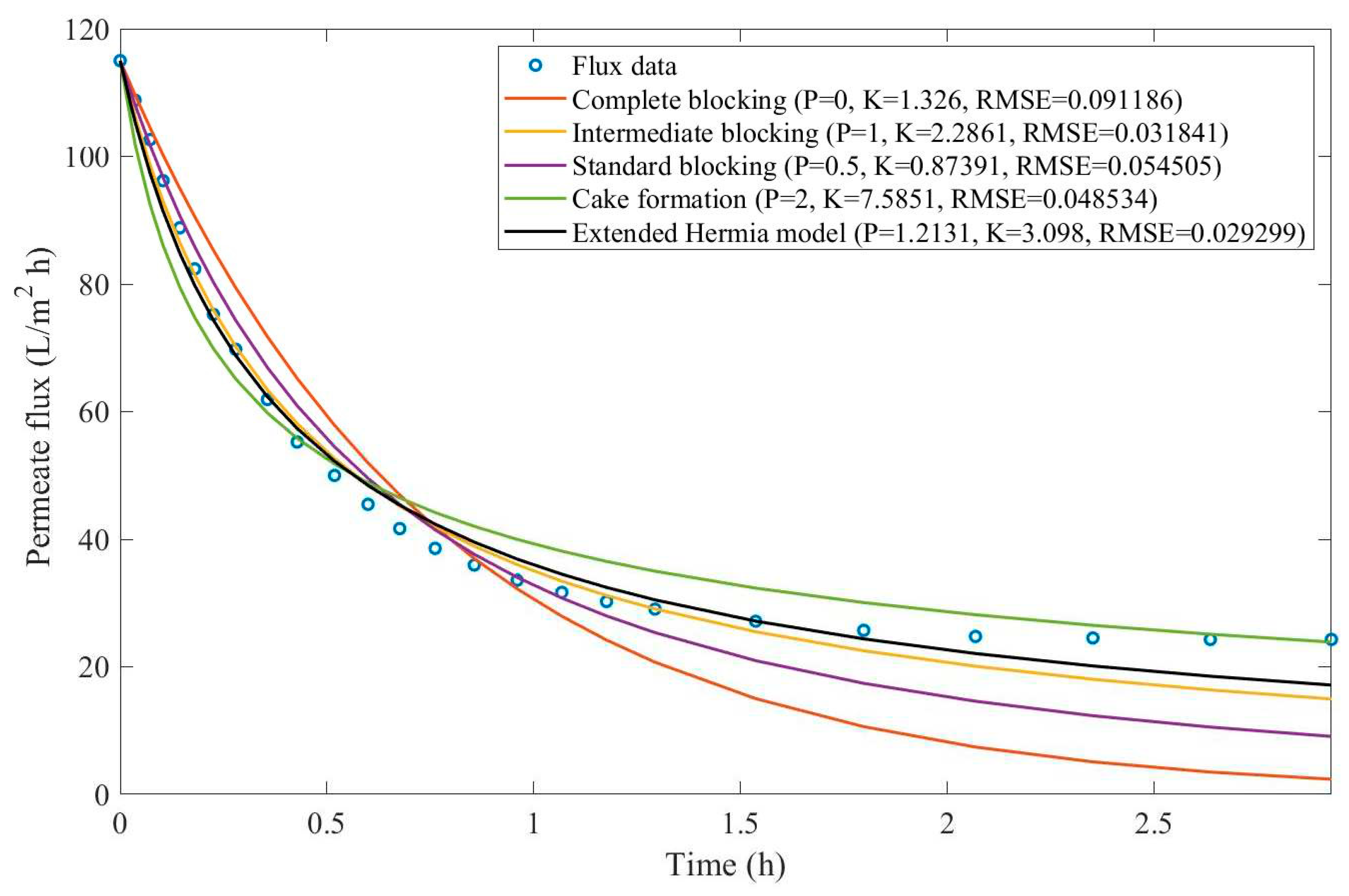
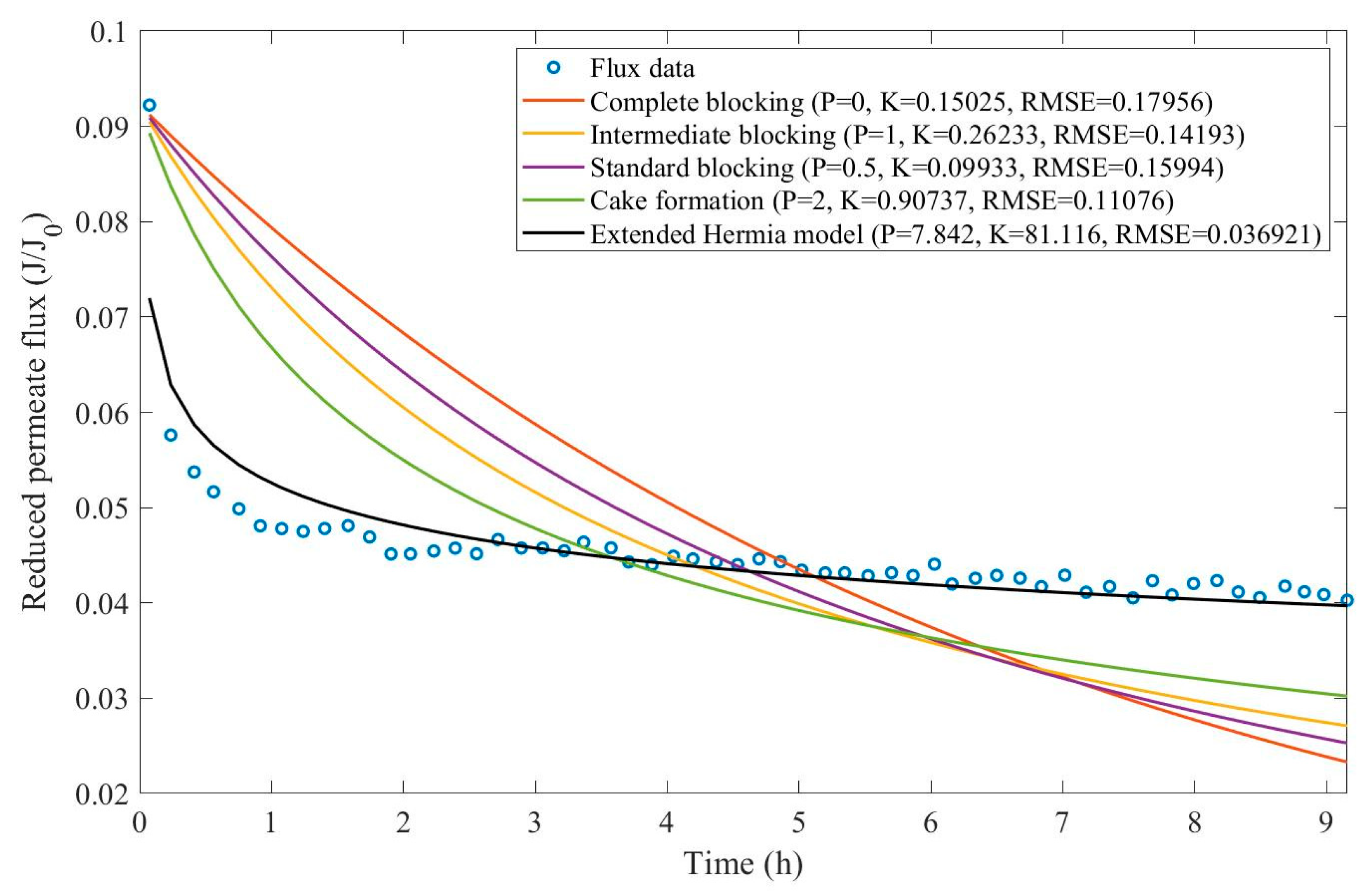
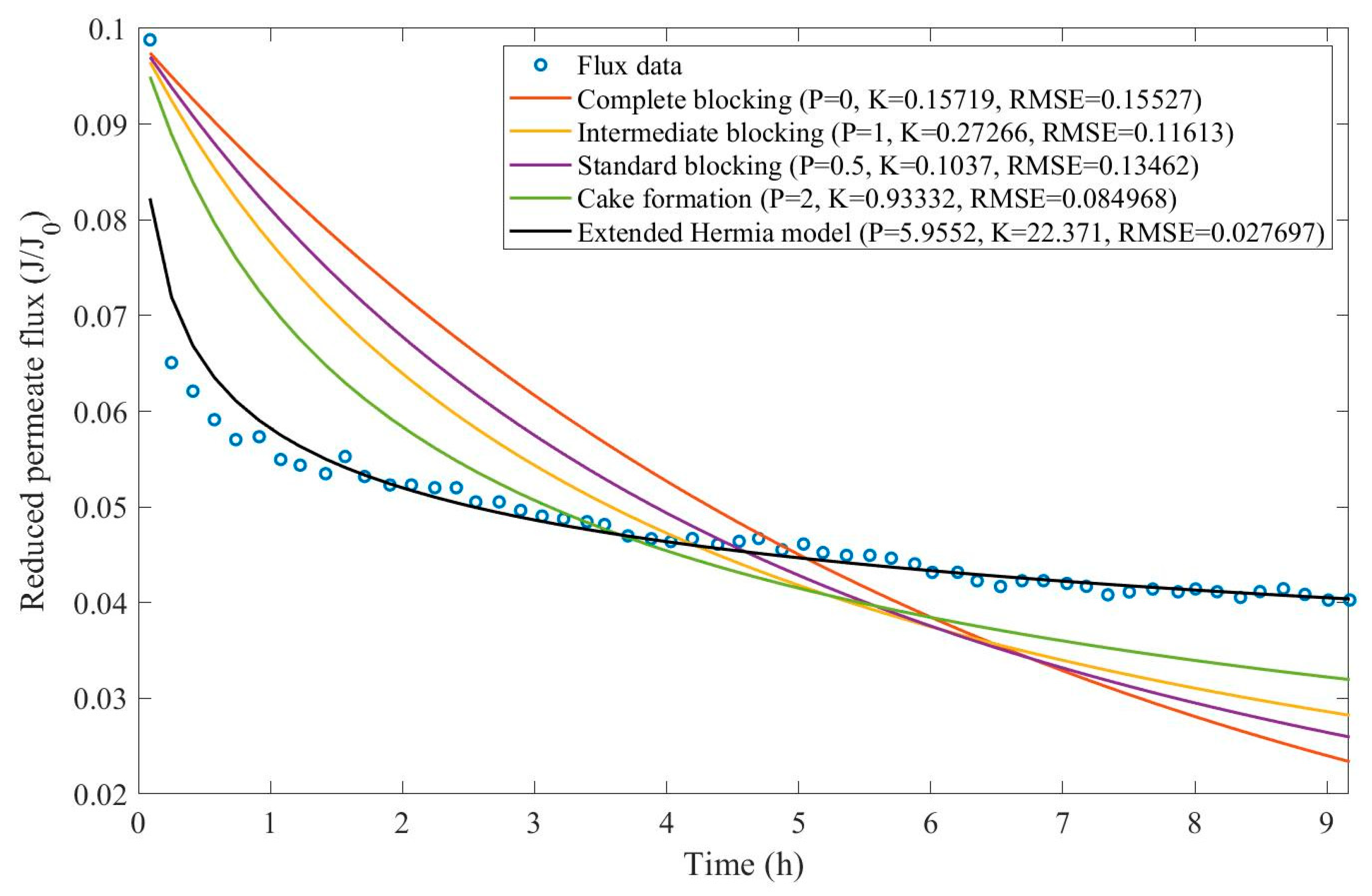
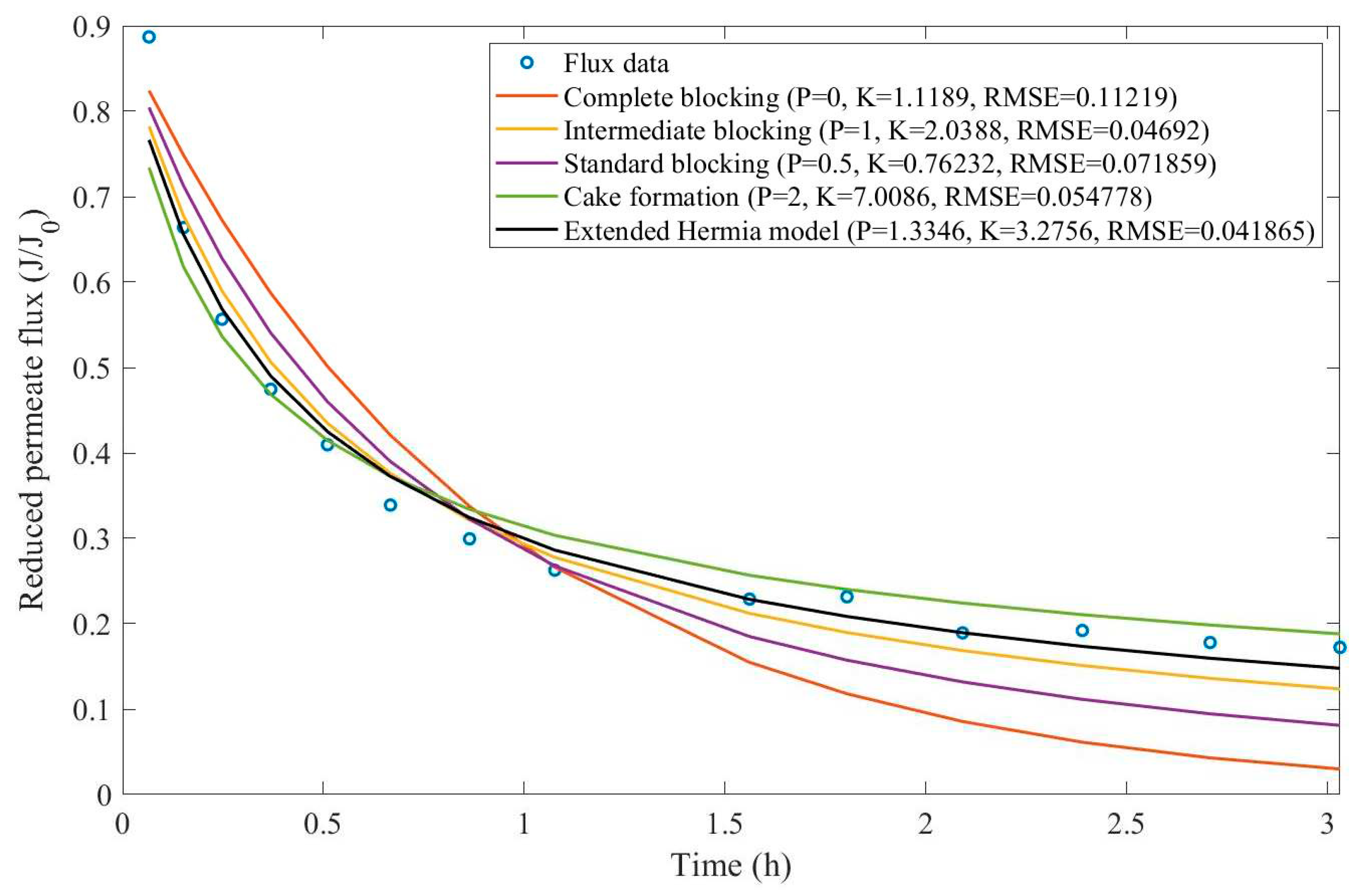
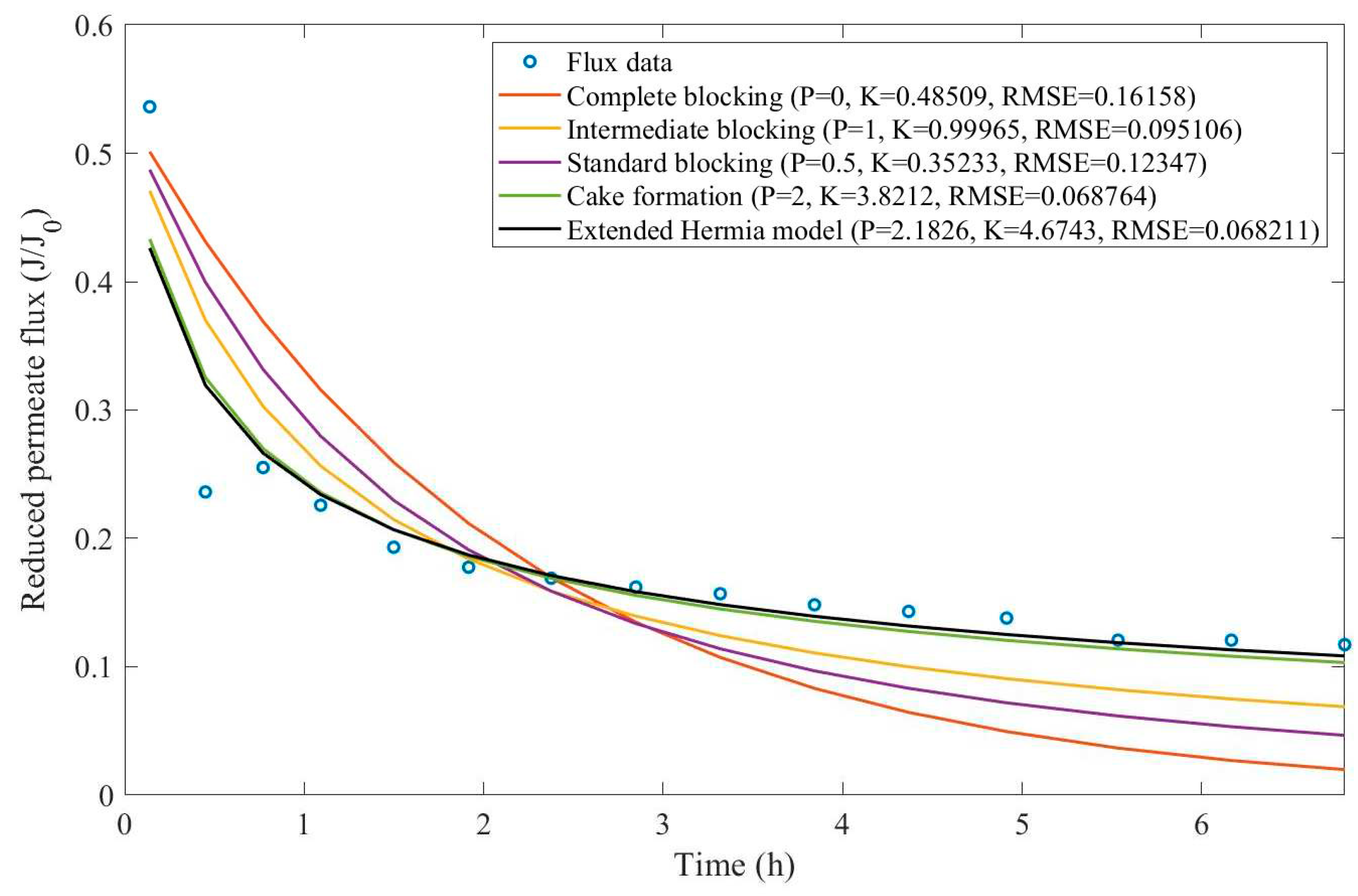
16]. We have recovered the data obtained throughout the experiments with CM08, CM05, and CM01 and performed the model fitting for all four pore-blocking mechanisms and the EHM. We have also isolated the data for different TMP conditions for microfiltration with CM05. These results can be found in
Appendix D (Figure D 9-Figure D 14),
Table 3,
Table 4,
Table 5, and
Table 6.
According to
Table 3 and
Table 4, the EHM has performed better than the four classic pore-blocking mechanisms (RMSE≤0.035), followed by cake formation (RMSE≤0.042). We have also noticed that the pore-blocking mechanism varies from membrane to membrane in the present example. Both CM08 and CM05 have a 1st-degree pore-blocking mechanism (between intermediate blocking and cake formation), while CM01 has a 2nd-degree blocking (between cake formation and a possible new type of pore-blocking). Meanwhile, the EMH half-life calculated for CM05 reveals that fouling doesn’t affect this membrane as much as it does CM08 and CM01, therefore this membrane has been chosen by Almandoz and coauthors for later tests [
16]. We have consolidated the data from these later tests and performed the same analysis. The regression results are presented in
Table 5 and
Table 6.
Taking into consideration
Table 5 and
Table 6, the best-performing models are EHM, cake formation, and intermediate blocking. At times intermediate blocking performs better than cake formation, yet the EHM still performs better than both. It is also interesting to point out that Figures have EHM with
, indicating that a mixed blocking mechanism between intermediate blocking and cake formation can happen simultaneously. In this case, it seems that an increase in TMP causes a slight shift in the most prevalent blocking mechanism from cake formation to intermediate blocking since
goes from 1.69 to 1.21. We have noticed that the middle ground between cake formation and intermediate blocking slightly increases the EHM half-life, which is the desired outcome when optimizing the filtration conditions.
Example 3. Model fitting for cross-flow hollow fiber ultrafiltration of oily effluent from a railway workshop
In a paper by Kurada and Tanmay [
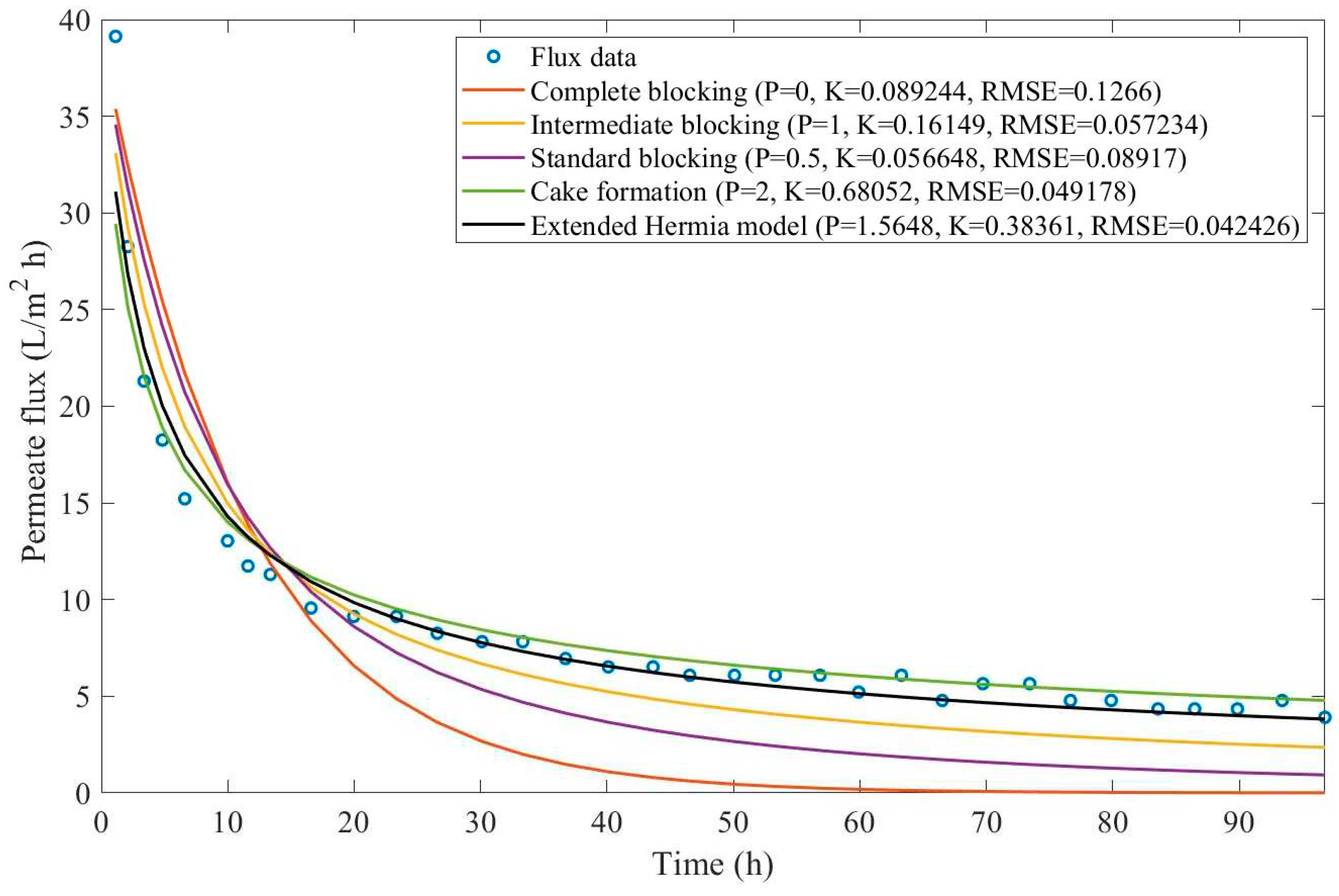
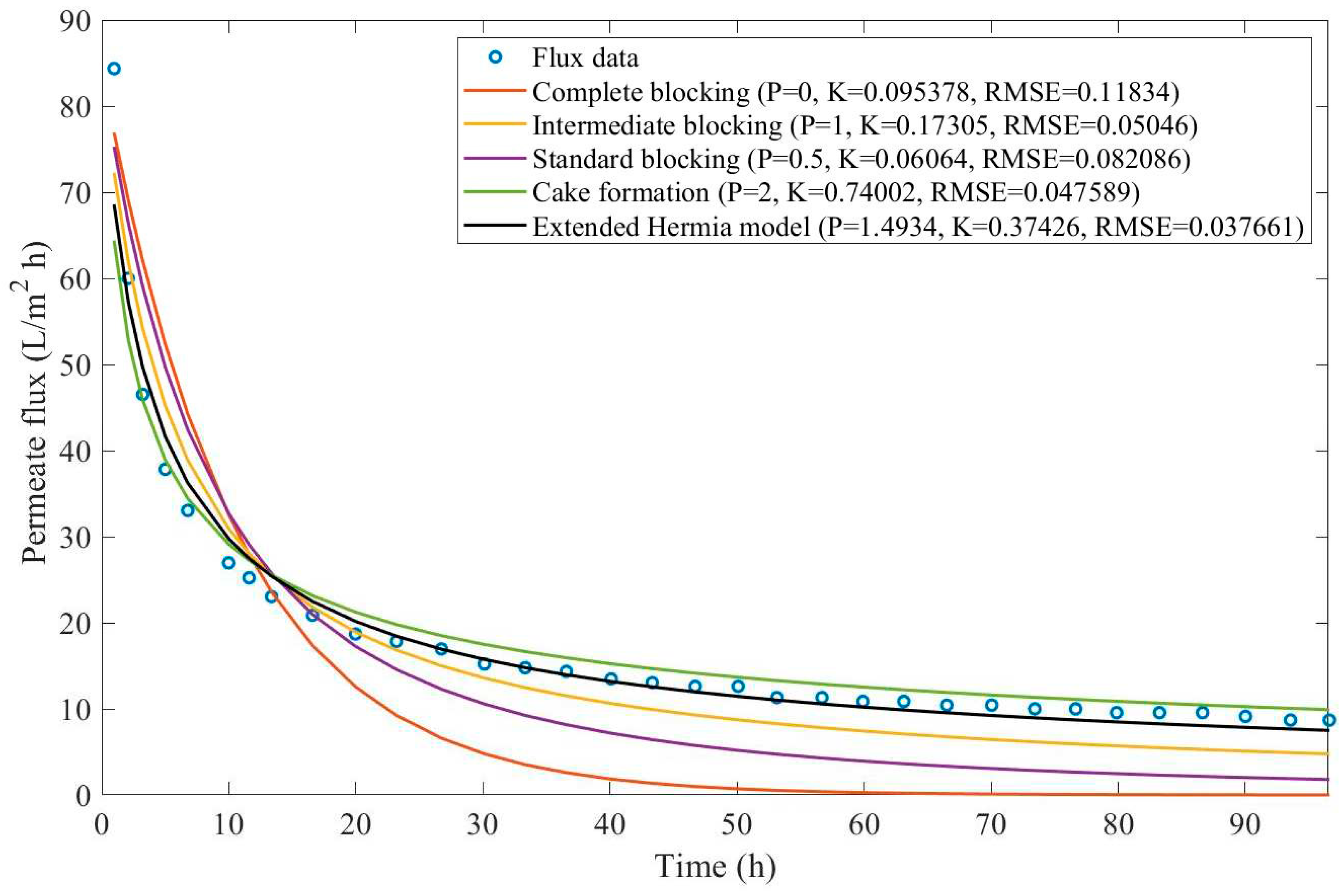
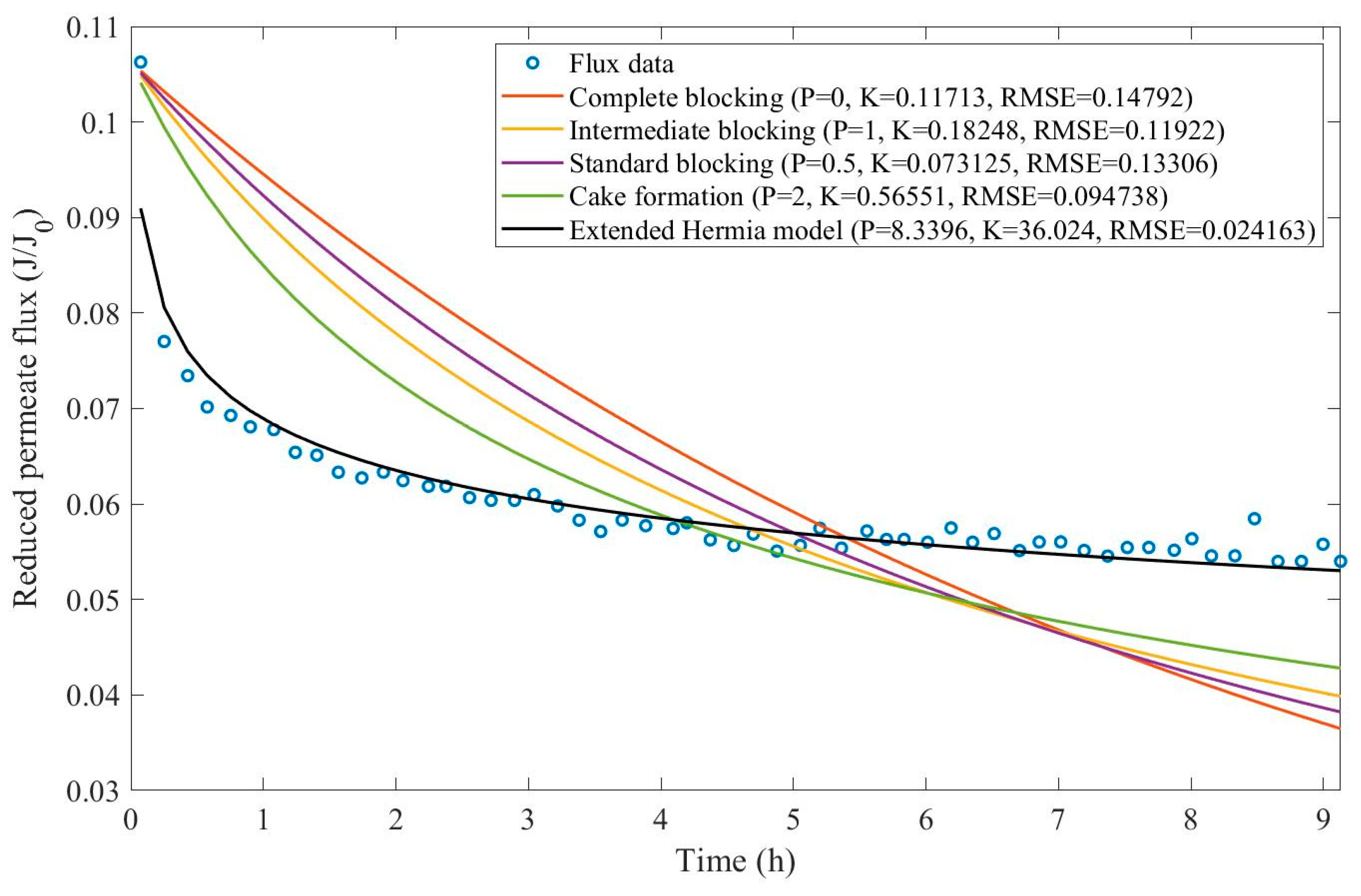
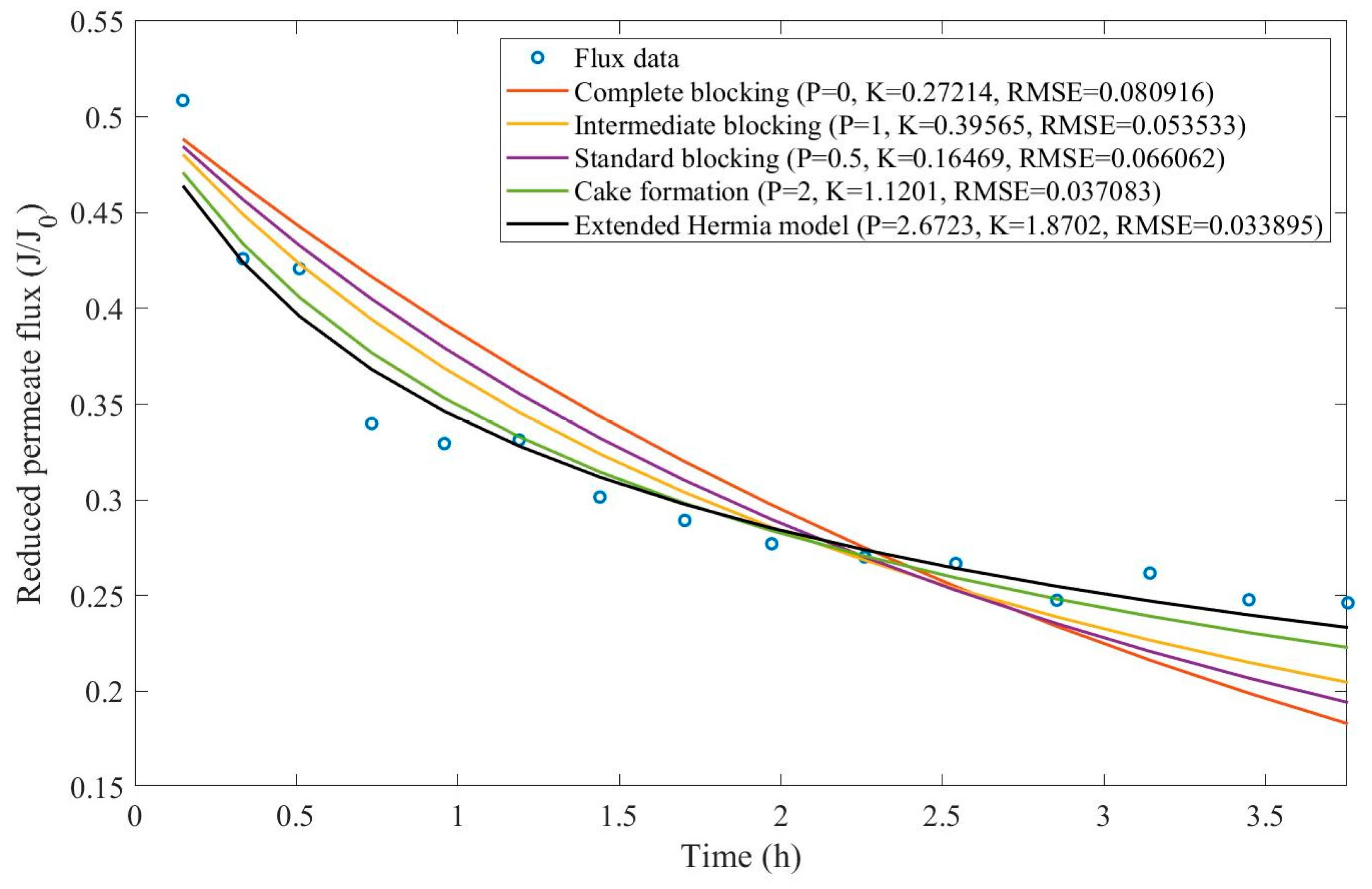
17], oily effluent containing dust, grease, and oil has been treated by a sand bed followed by a cross-flow ultrafiltration hollow fiber membrane. Their experimental work involved changing both TMP (21-104 kPa) and CFR (14-40 L/min) and evaluating the aftermath, such as flux reduction and cake layer thickness. We have extracted the data obtained by Kurada and Tanmay and applied the same techniques presented in Examples 1 and 2. The regression results and EHM parameters can be found in
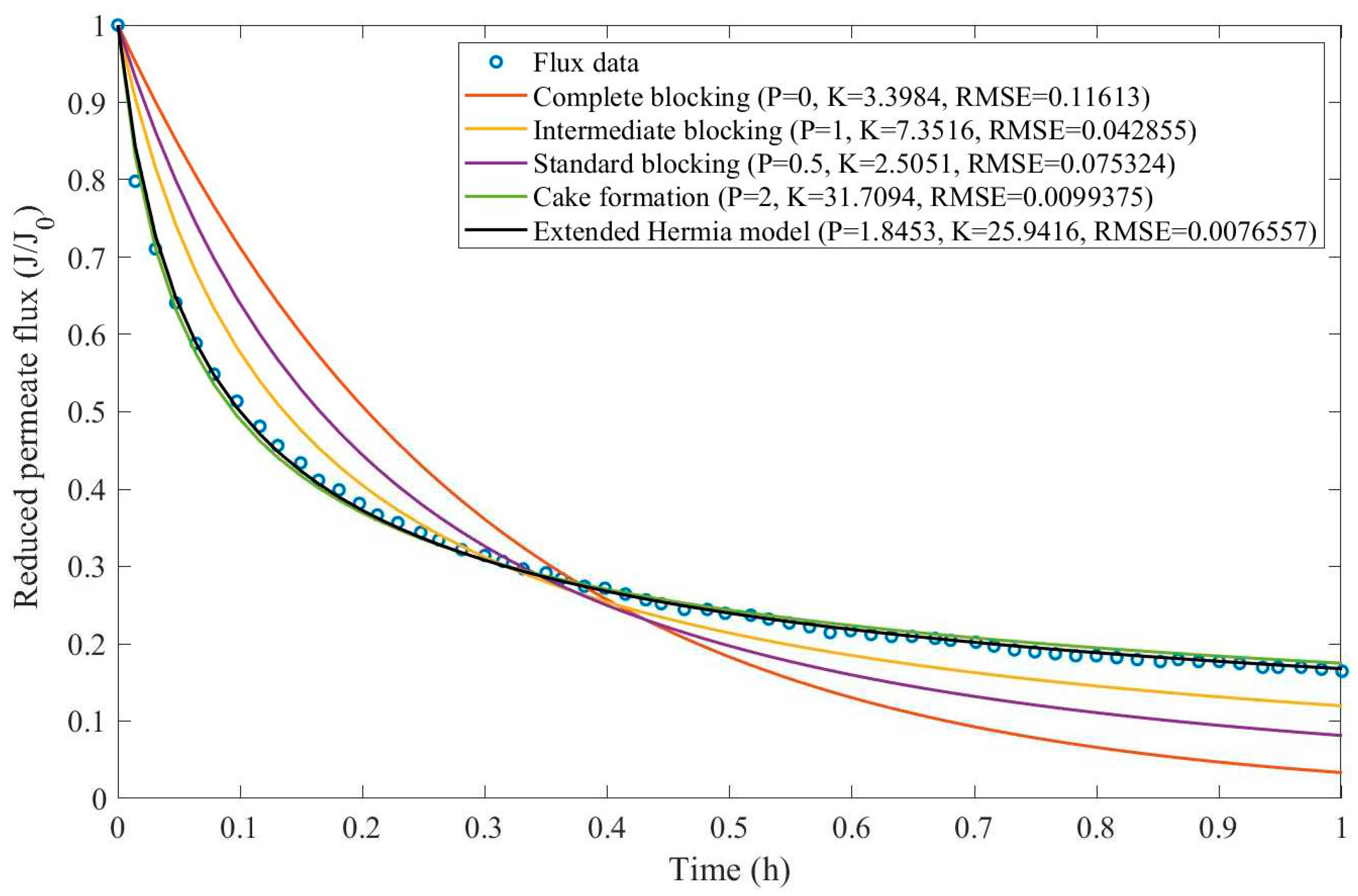
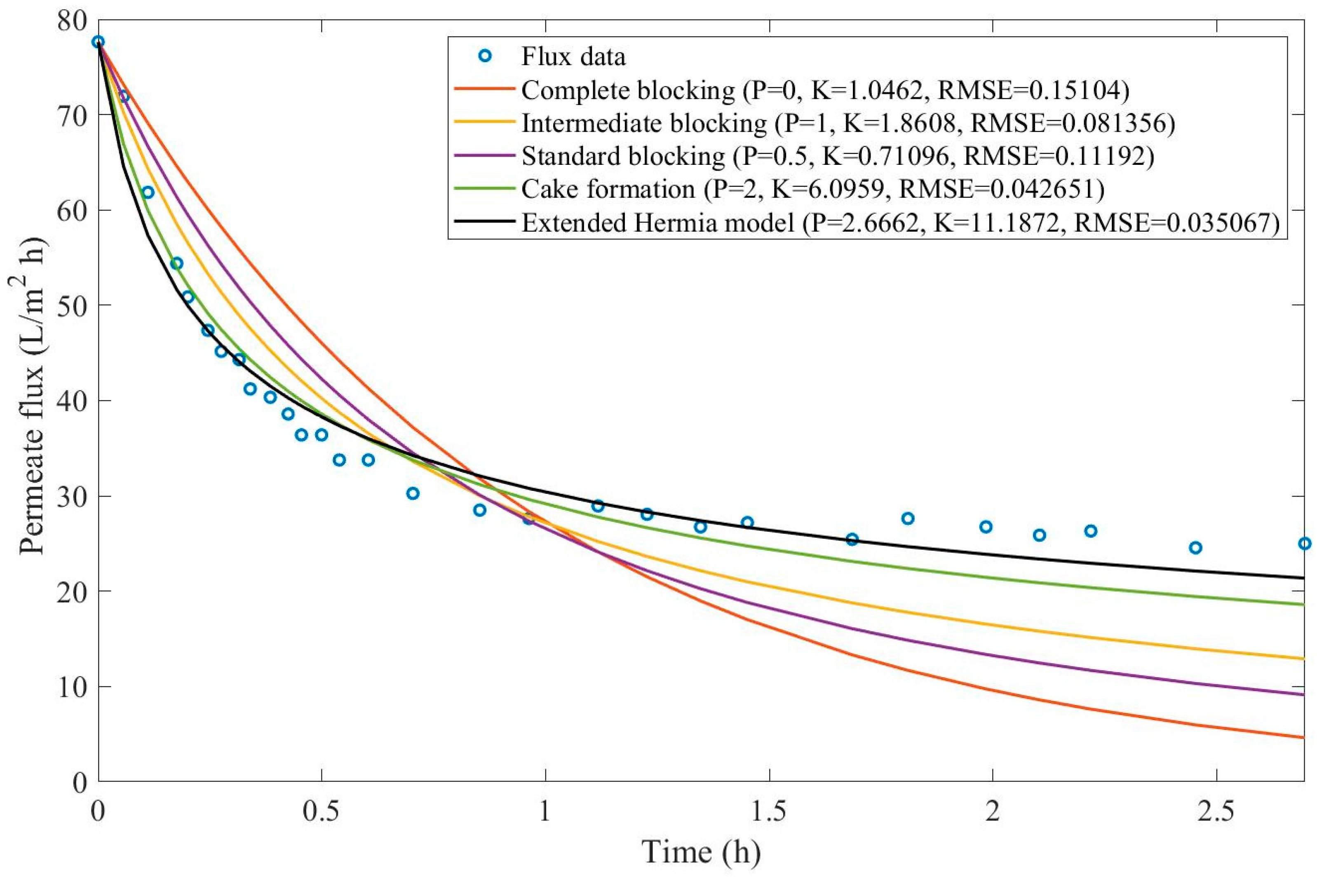
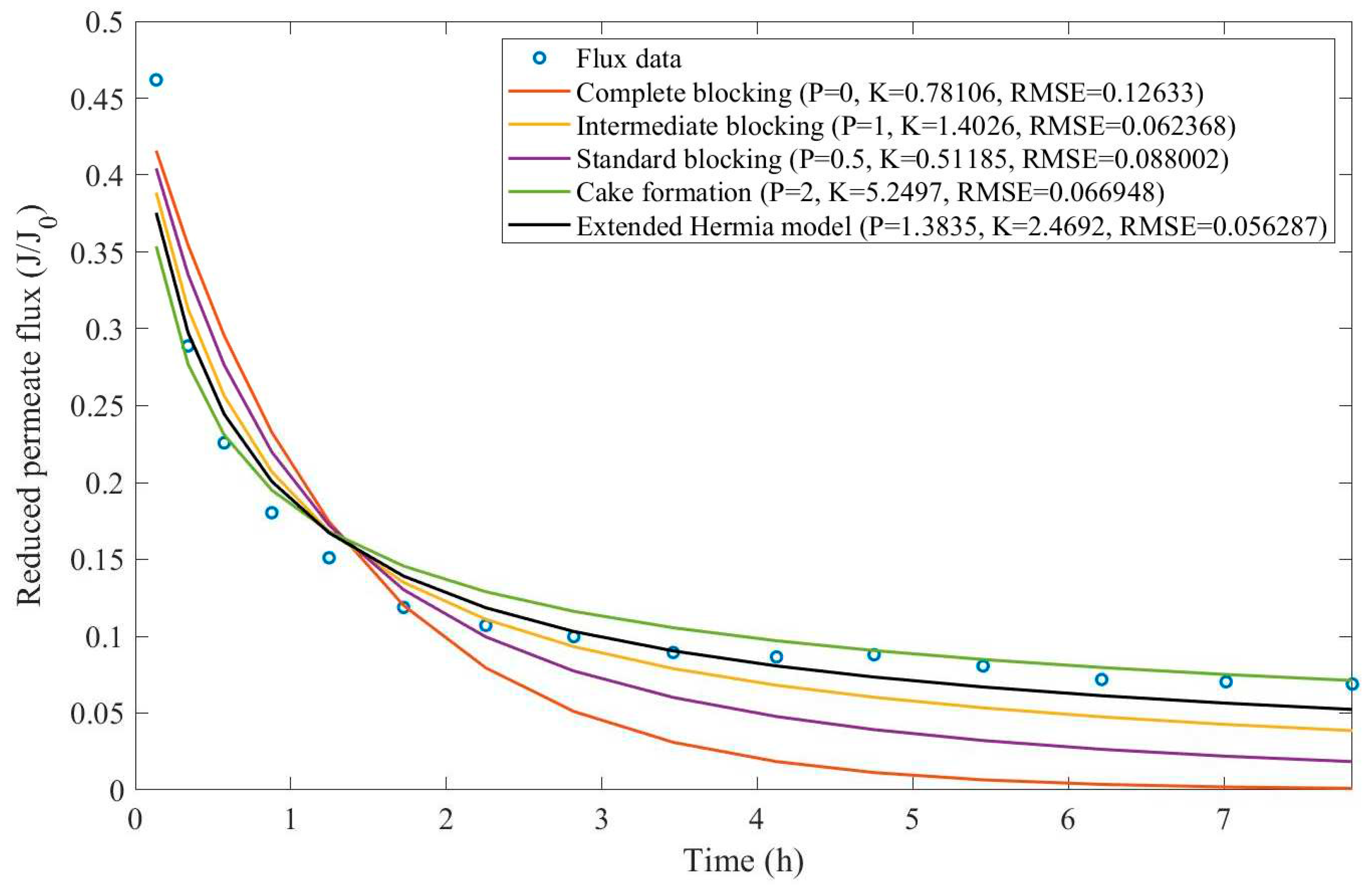
Appendix D (Figure D 15-Figure D 23),
Table 7, and
Table 8.
Based on the regressions presented in
Table 7 and
Table 8, we have observed a very similar behavior to Example 2, in which the best performance was credited to the EHM (RMSE≤0.042). cake formation and intermediate blocking have also performed well (RMSE≤0.049 and ≤0.057 respectively). In Example 2 we have pointed out that an increase in TMP changes the most prevalent pore-blocking mechanism from cake formation into intermediate blocking because
had its value decreased. The same effect is also present here but only for a CFR of 14 L/min. For CFRs of 28 and 40 L/min, is seems that
behaves differently, increasing or decreasing with TMP. Changes in CFR while maintaining TMP constant also seem to have the same effect. Therefore, for the present Example, it seems that significant changes in TMP and CFR do not change the pore-blocking mechanism considerably. We have also noticed that decreasing both TMP and CFR leads to an increase in EHM half-life since, in this case,
tends to cake formation and lower values of TMP and CFR prevent the cake layer thickness from increasing as rapidly.
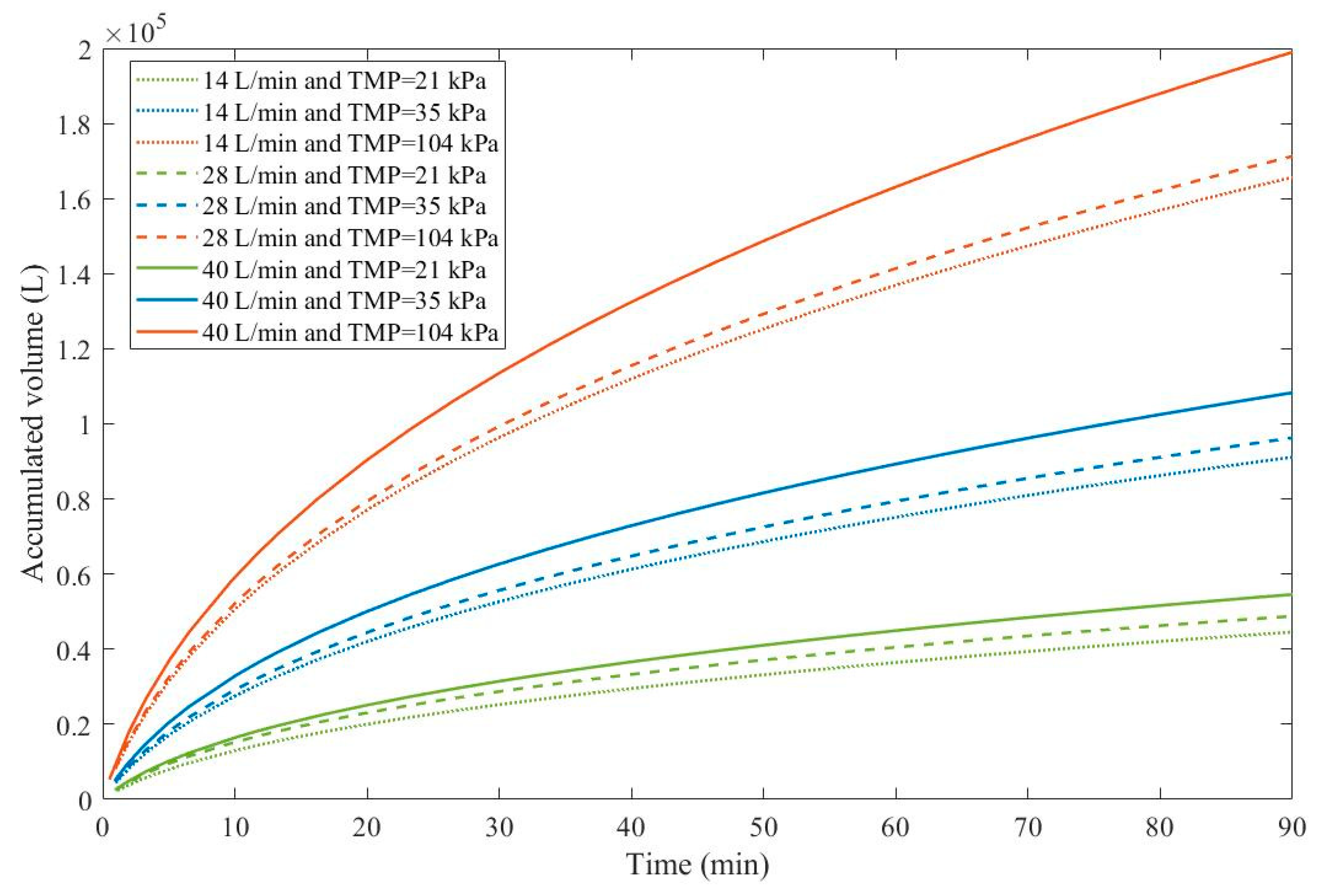
As a consequence, rather than looking at which experimental conditions lead to the most advantageous pore-blocking mechanism, we have to analyze which conditions result in higher values of
and how it affects the accumulated permeate volume given in Eq. 22 (
Figure 3).
We have color-coded
Figure 3 for a better understanding. TMP is represented in different colors, green for 21 kPa, blue for 35 kPa, and red for 104 kPa. Full lines represent 40 L/min, discontinued lines represent 28 L/min and dotted lines represent 14 L/min. Through
Figure 3, we have noticed that an increase in TMP causes a general increase in the accumulated volume for all CFRs. For all TMPs we have observed an increase in CFR also causes an increase in the accumulated volume. Therefore, in Example 3, higher TMPs and CFRs are advantageous. It is important to point out that this effect is only possible because changes in both TMP and CFR do not change the blocking mechanism greatly, as shown in
Table 7. For instance, in Example 4 we demonstrate that an increase in crossflow velocity can either increase or decrease the accumulated volume depending on the blocking mechanism.
Example 4. Model fitting for ultrafiltration of alkali/surfactant/polymer flooding wastewater
In an experimental work by Ren et al., ultrafiltration was used to treat Alkali/surfactant/polymer (ASP) flooding wastewater, a commonly produced effluent in enhanced oil extraction processes that needs to be properly treated before reuse due to the potential threat of formation damage. In this study, the operating parameters were modified to research their effects on membrane fouling, which aimed to optimize the filtration conditions to minimize the effect of flux reduction. These parameters included TMP (2.12-2.79 bar) and CFV (0.75-3.00 m/s), with the ideal conditions being a TMP of 2.12 bar and CFV of 3.00 m/s [
18].
We have recovered the flux data obtained by Ren et al. and performed the model fitting for all four classic pore-blocking mechanisms and the EHM. These results are presented in
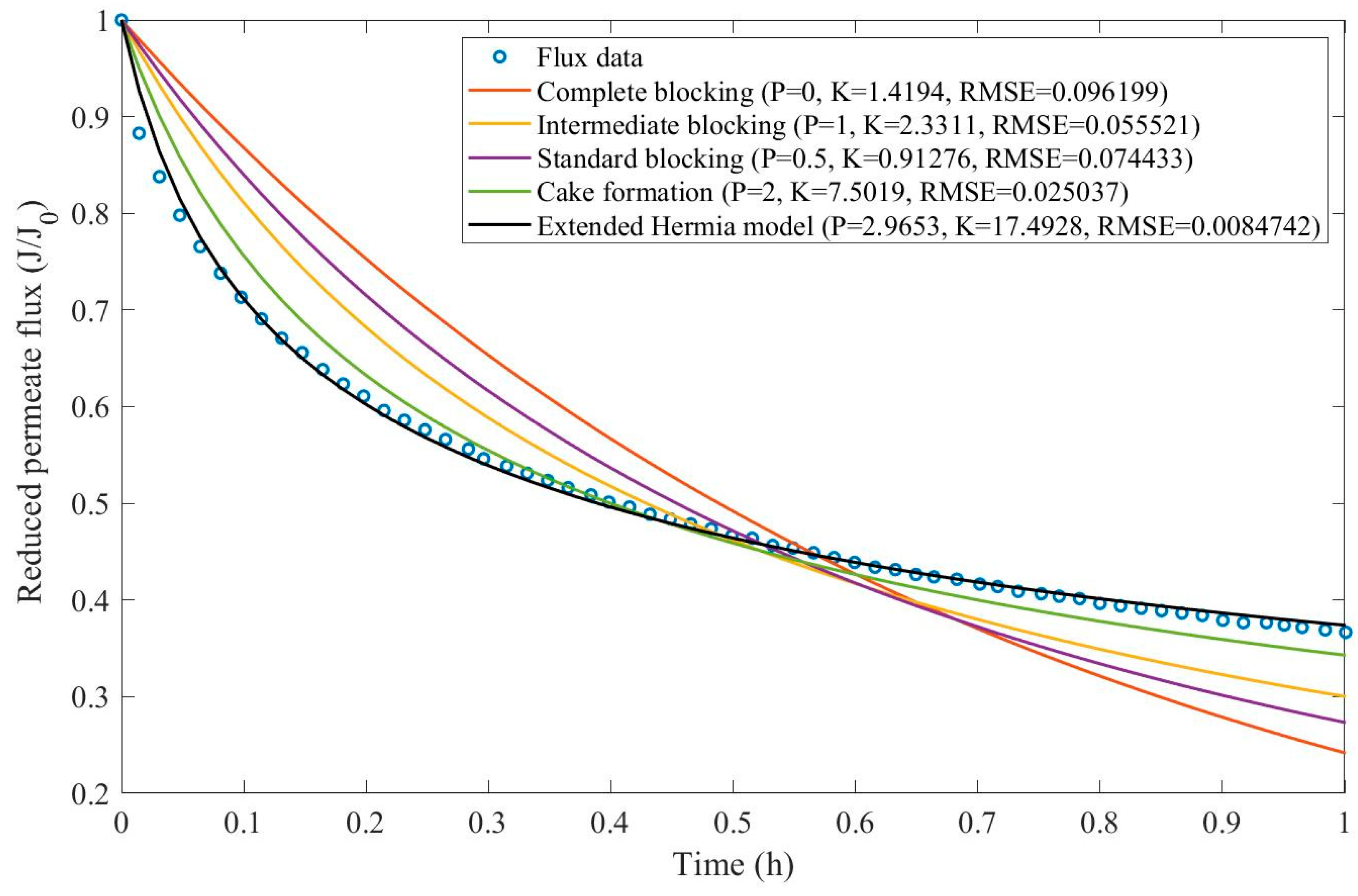
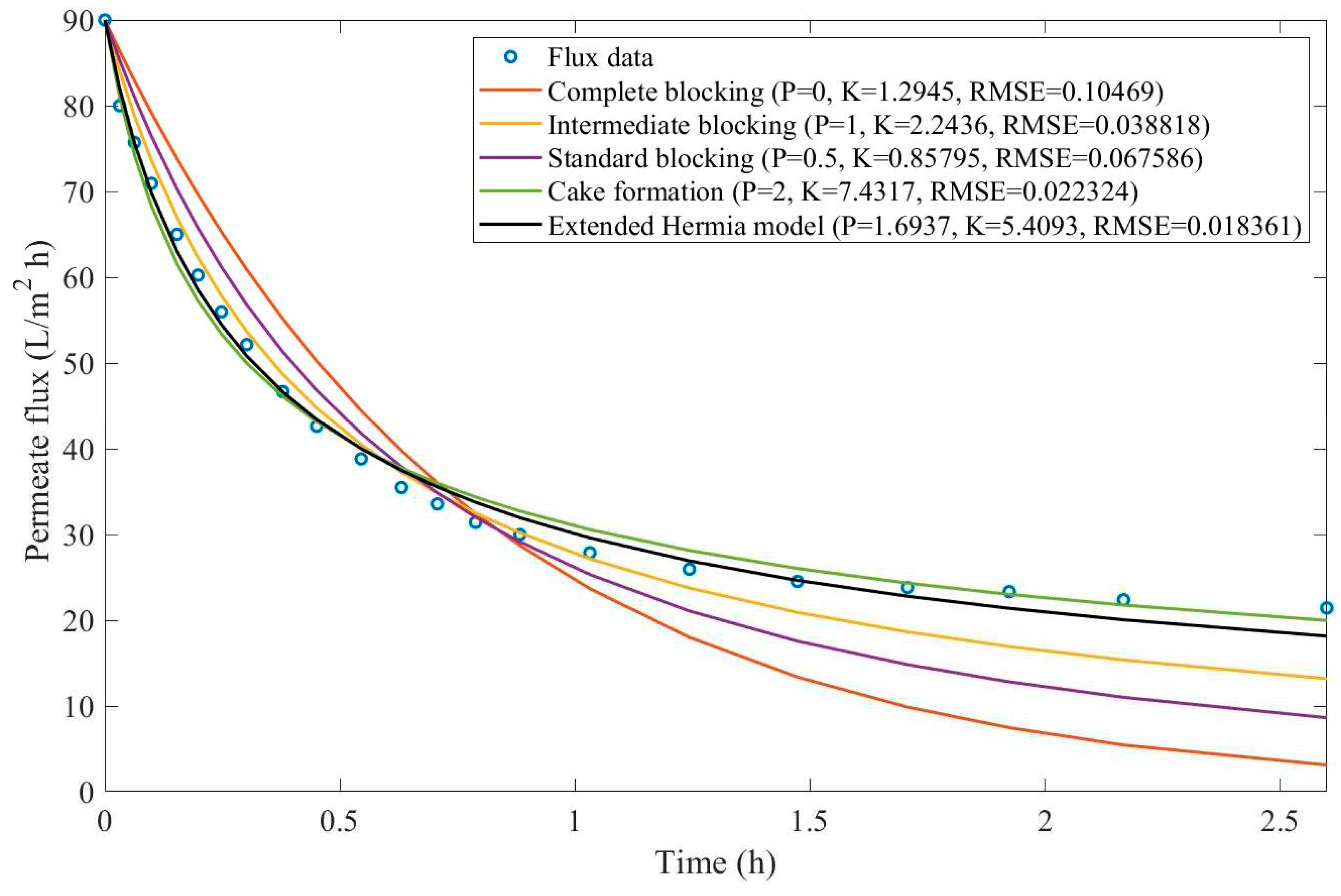
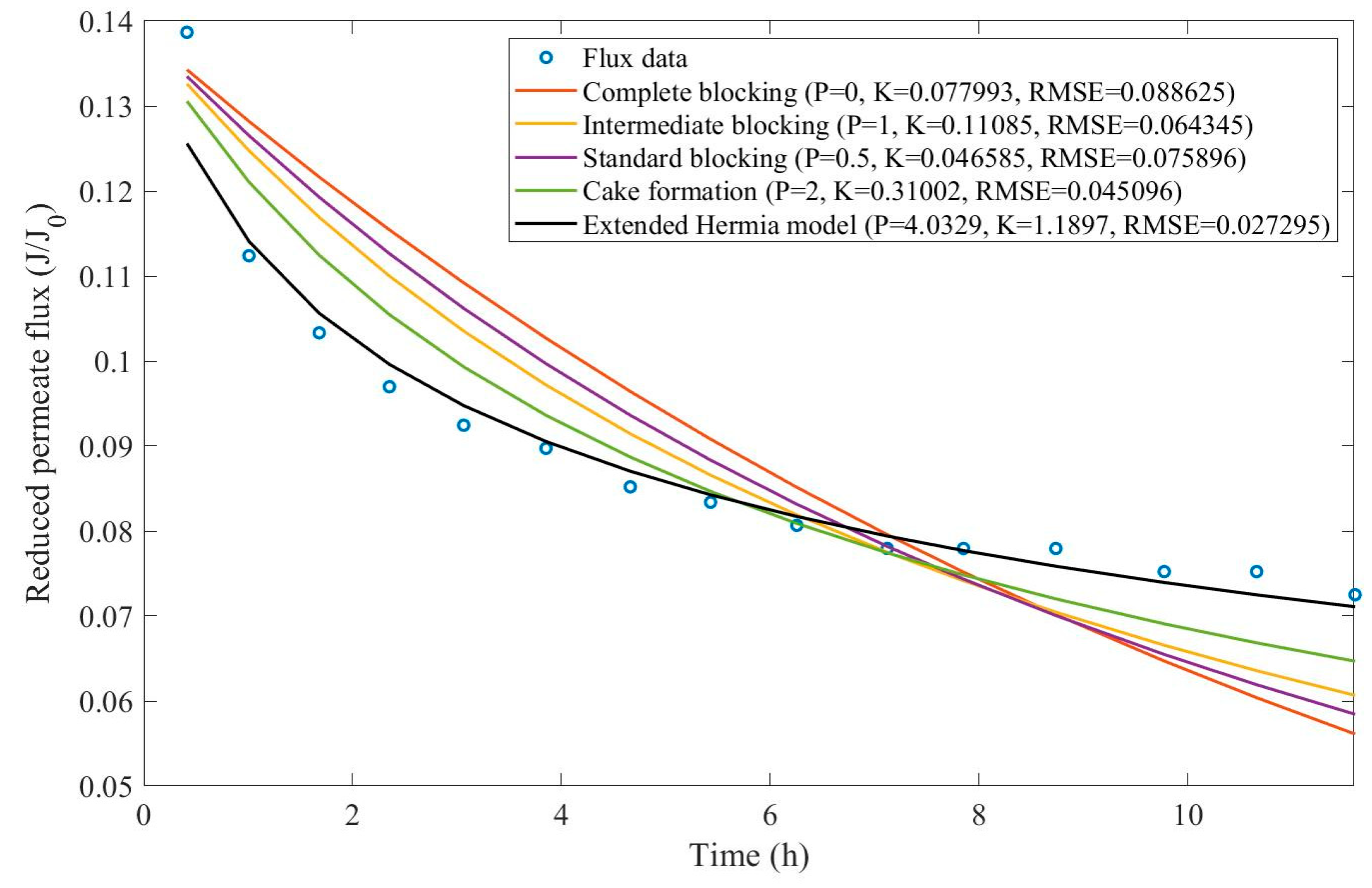
Appendix D (Figure D 24-Figure D 29),
Table 9,
Table 10,
Table 11, and
Table 12.
According to
Table 9 and
Table 10, across all the model fittings, the EHM presents the best data fit (RMSE≤0.015), as the other pore-blocking mechanisms, do not seem to fit the data accurately. We have noticed that an increase in TMP causes a decrease in the value of
changing the pore blocking mechanism from a 9th-degree to a 4th-degree. The present example was included to demonstrate that even though the EHM fits the data well, it is important to exercise caution when doing so. The EHM half-life, when calculated using Eq. 20, yields results that are not physically accurate. Since the data recovered from Ren et al. does not include
in Figure D 24-Figure D 29, the values obtained for
and
do not represent values
. Therefore, the use of Eq. 20 extrapolates the model for points that were not included in the regression, which results in EHM half-life values that are non-representative. The same regressions have been performed on the experimental tests at 2.20 bar with varying CFV (0.75-3.00 m/s) (
Table 11 and
Table 12).
Taking into account
Table 11 and
Table 12, we have observed that the EHM had a better performance (RMSE≤0.037) when compared to the four original pore-blocking mechanisms. For the present example, it seems that changes in CFV affect
greatly, changing between 5th-degree and 9th-degree mechanisms. Once again, the EHM half-life is not physically representative because it is extrapolating the model, such as in
Table 9, therefore if this happens in the following Examples (Example 5 and Example 6), the EHM half-life will be referred to as non-applicable (N/A). One alternative to better rank the filtration conditions to optimize the process is to use Eq. 22. By calculating the accumulated volume of permeate, it is possible to obtain a function of
and
, making it possible to rank the filtration conditions through accumulated volume maximization.
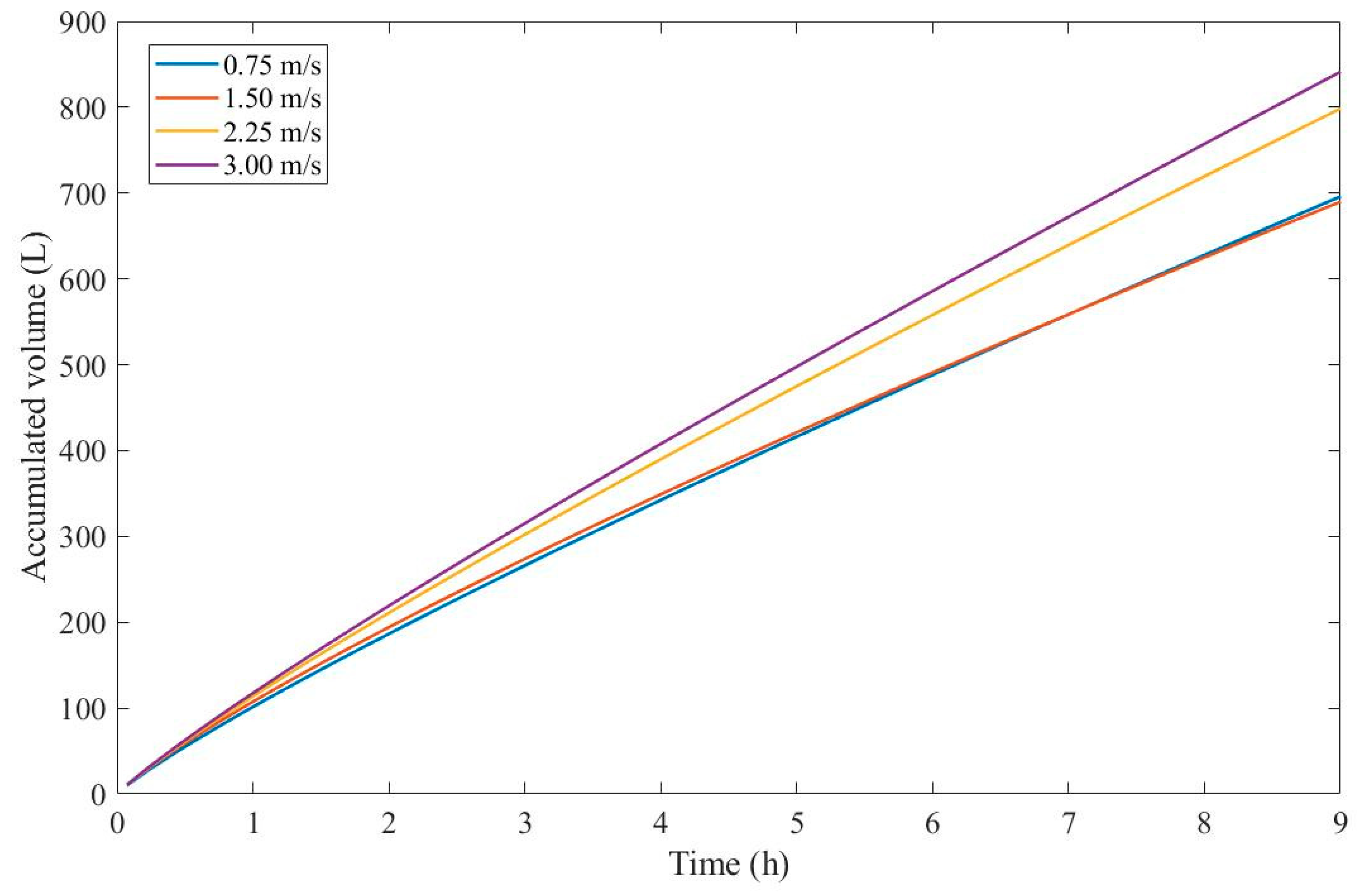
Figure 4 presents the accumulated volume calculated using Eq. 22 assuming a
and
for all CFVs.
Through
Figure 4, we have calculated that an accumulated volume for a CFV of 3.00 m/s yields better results, therefore a 9th-degree pore-blocking mechanism is favorable in this context. Due to the non-linear nature of Eq. 22, a higher CFV will not always provide higher values for the accumulated volume, as shown in
Figure 4 for CFVs of 0.75 and 1.50 m/s. At one point both of their curves meet, which means that, at times, fouling will affect the membrane to such a degree that lower CFVs would yield a higher permeate production.
Example 5. Model fitting for ultrafiltration of bovine serum albumin solutions
Aiming to decrease the effects of fouling in iron oxide ultrafiltration membranes, Storms and collaborators coated these ceramic membranes with poly(sulfobetaine methacrylate) (polySBMA), a superhydrophilic zwitterionic polymer and investigated whether this modification was helpful towards flux reduction. Albumin solutions were filtered at a TMP of 103.421 kPa in three fouling stages for both uncoated and coated membranes, such that washings were performed between stages [
19].
We have recovered the experimental data obtained for the three fouling stages for both uncoated and coated membranes. The same model fitting performed in Examples 1-Example 4 was also applied to the present example. The regressions obtained are presented in
Appendix D (Figure D 30-Figure D 35),
Table 13, and
Table 14.
By the results presented in
Table 13 and
Table 14, we have demonstrated that the EHM performs better than the original four pore-blocking mechanisms since it has an RMSE≤0.068. According to the values obtained for
, the addition of polybag changes the pore-blocking mechanism for the first fouling stage, which starts with a prevalent mechanism of intermediate blocking and changes slightly to cake formation since
goes from 1.33 to 1.42. This change is further supported by the second fouling stage, in which
for the uncoated membrane and 2.18 for the coated membrane. The third fouling stage shows that the uncoated membrane has a big shift in the pore-blocking mechanism going from a mainly intermediate blocking (1st-degree) to a 4th-degree. In contrast, the third fouling stage for the coated membrane stays mainly a cake formation mechanism (2nd-degree).
We have also observed an increase in EHM half-life in the first and second fouling stages, which indicated that the addition of polySBMA does mitigate fouling to a certain degree. It is also interesting to point out that the polySBMA coating seems to cause a cake formation mechanism, which, in this case, is advantageous since it increases the EHM half-life and the amount of permeate obtained per filtration batch.
Example 6. Model fitting for ultrafiltration of nanoparticles from polishing wastewater
In a series of ultrafiltration experiments at a laboratory scale conducted by Ohanessian et al., chemical mechanical polishing wastewater filtration was carried out to optimize and validate fouling models. Two types of experiments have been performed: dead-end filtration at a TMP of 0.4 bar and crossflow filtration at a TMP of 0.3 bar. Different concentrations were evaluated for both, 97, 251, and 657 mgNPs/L (milligrams of nanoparticles per liter) for dead-end filtration and 332, 572, and 2600 mgNPs/L for crossflow filtration [
20].
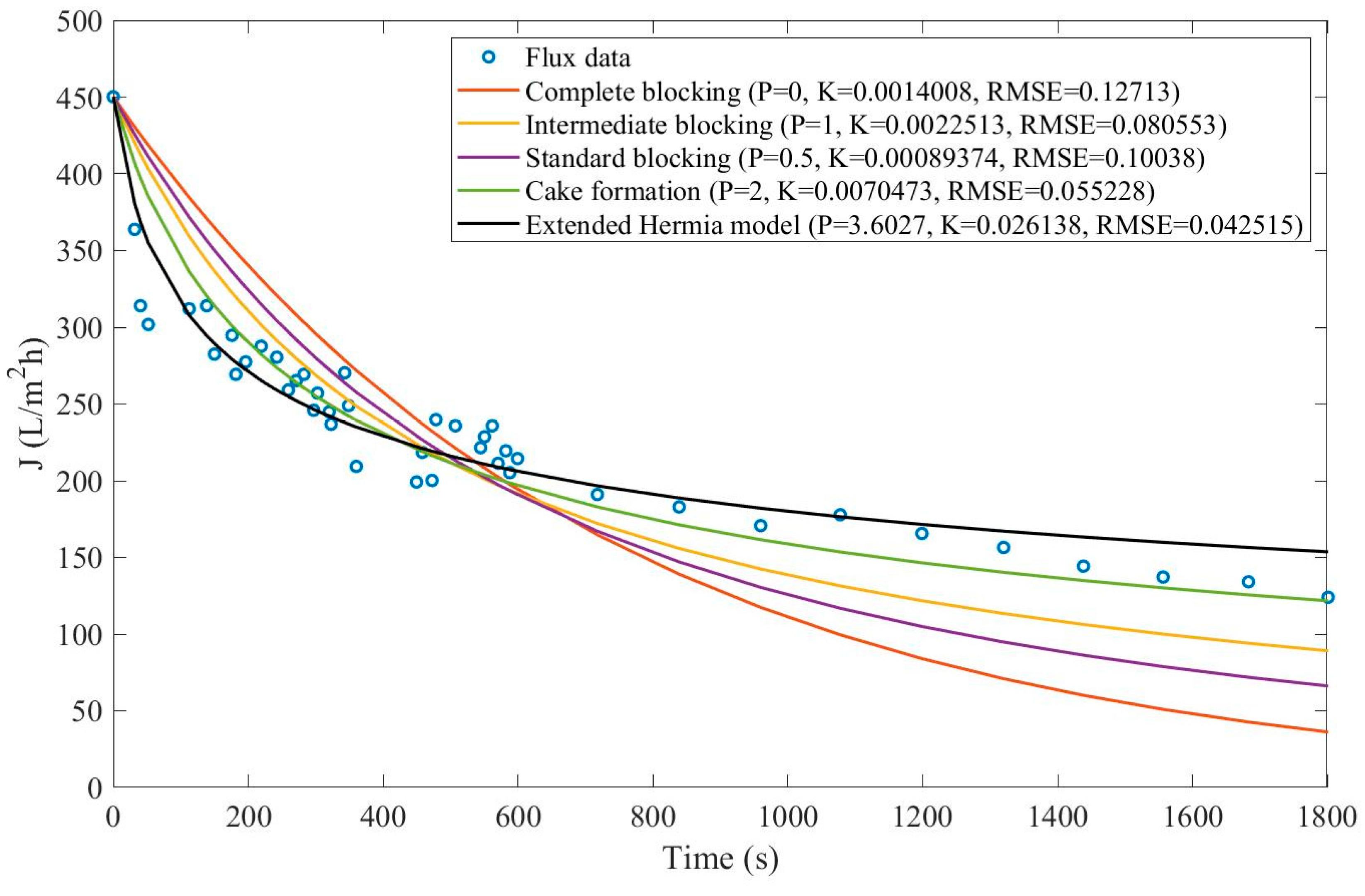
We have recovered the data from the dead-end filtration experiments and performed the model fitting for all original pore-blocking mechanisms, as well as the EHM. The regression results are presented in
Appendix D (Figure D 36-Figure D 38),
Table 15, and
Table 16.
According to
Table 15 and
Table 16, throughout the experiment, the EHM has consistently performed better than the original pore-blocking mechanisms (RMSE≤0.042) followed closely by the cake formation mechanism (RMSE≤0.055). We have also noticed that an increase in the concentration of nanoparticles non-linearly changes the pore-blocking mechanism, starting at a 3rd-degree and moving to a mostly cake formation mechanism (2nd-degree). In contrast, a further increase in nanoparticle concentration does the opposite, changing from mostly cake formation to a mixed pore-blocking mechanism of cake formation and 3rd-degree blocking.
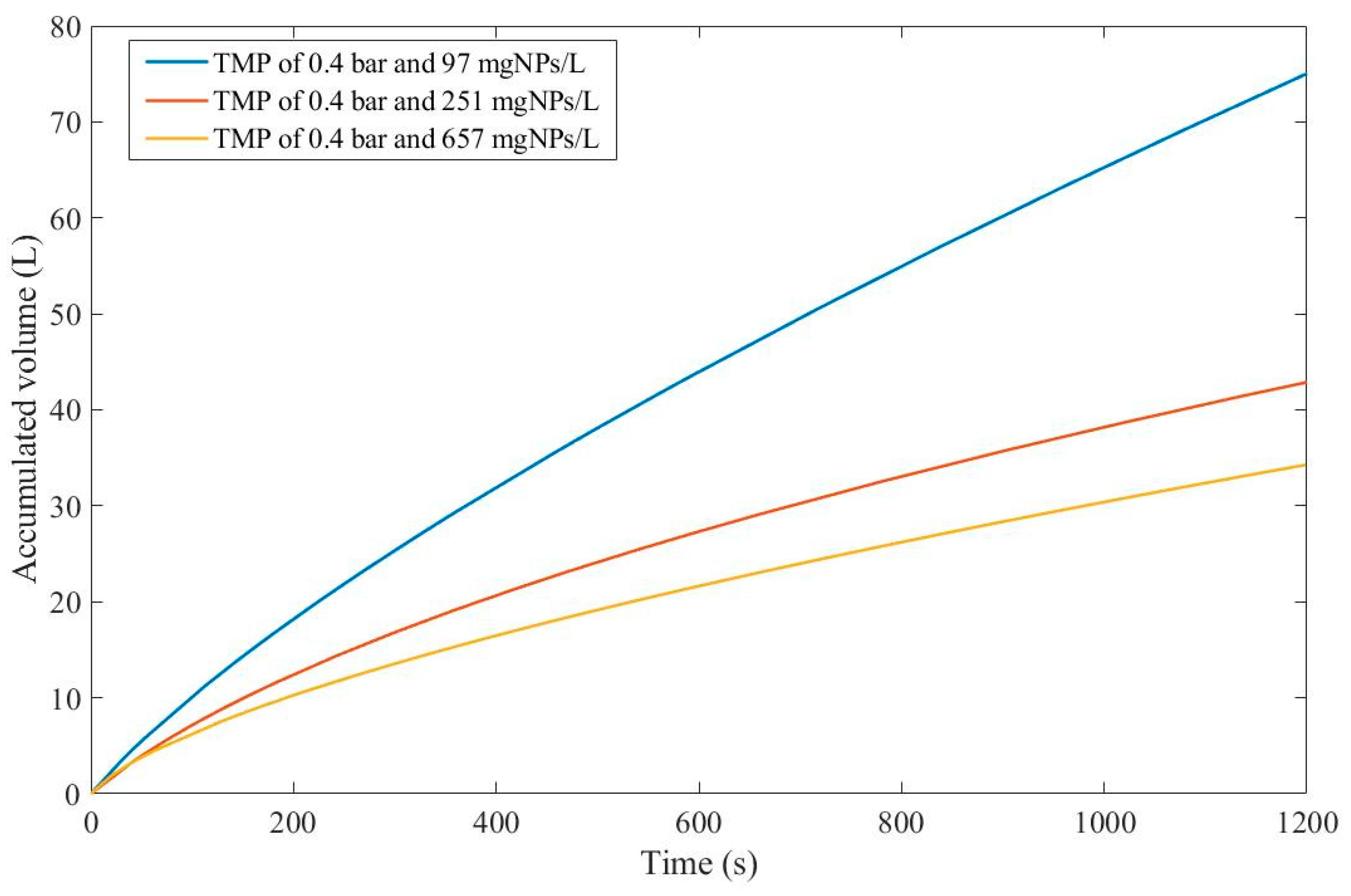
Taking into account the EHM half-life, it seems that an increase in nanoparticle concentration is directly correlated with a decrease in half-life, which indicates that fouling has a bigger effect at higher concentrations. It is important to point out that the flux data presented in Figure D 36-Figure D 38 have different values of
for each concentration, therefore, to better classify which pore-blocking mechanism is the most advantageous in Example 6, we have used Eq. 22 and the regression results to calculate the accumulated permeate volume (
Figure 5) assuming
.
Even though
for 657 mgNPs/L is greater than
for 97 mgNPs/L,
Figure 5 shows that the accumulated volume obtained through a concentration of 97 mgNPs/L is far greater than for higher concentrations. This implies that the effects of fouling are more pronounced for higher concentrations as demonstrated through EHM half-life. Therefore, we can conclude that, in Example 6, a 3
rd-degree pore-blocking mechanism at lower concentrations for dead-end ultrafiltration of nanoparticles at 0.4 bar is advantageous.