1. Introduction
The chondrichthyan lineage separated from the osteichthyan line at least 440 million years ago (Coates et al. 2018; Andreev et al. 2020) and has been evolving separately ever since into a lineage that is very different from other vertebrates. Along with their cartilaginous skeletons, for example, the fossil record shows that sharks were already using internal fertilization 390 million years ago (Brazeau & Friedman 2014). Their behaviour in the wild also presents great differences. While aggression is common in the osteichthyan line, for example, fighting among sharks has not been documented (e.g. Allee & Dickinson 1954; Hobson 1963; Myrberg & Gruber 1974; Johnson 1978; Gruber et al. 1988; Klimley 1982, 1988). Studies have identified what appears to be warning displays in social contexts (Johnson & Nelson 1973; Sperone et al. 2010; Klimley 2023 a,b,c,; Klimley et al. 2023 in prep) which take different forms in different species, and which are unique to elasmobranchs. Some fall under the definition of agonistic displays; for a review see Martin (2007). Others are more difficult to categorize (Porcher 2021). These suggest that shark behaviour may be more complex than was formerly thought, for shark science has been strongly influenced by fisheries and by the idea that sharks, as an ancient line, are unintelligent and lacking in behavioural complexity (Castro 2016). However, very few submarine ethological studies have been done, not only due to fear of sharks (Johnson & Nelson 1973; Gruber & Myrberg 1977; Randall & Helfman 1978; Klimley 2023a in prep), but also because they are fast moving and aquatic (Gruber & Myrberg 1978; Castro 2016).
Northcutt (1977, 1978) found that the development and comparative size of the elasmobranch brain are comparable with those of birds and mammals, while large relative brain size is positively correlated with complexity of ecological behaviour and cognitive capacities in other vertebrates. Guttridge et al. (2009) reviewed learning reported in earlier studies of elasmobranchs, and since then there has been more interest in intelligent elasmobranch behaviour. They have been found to use cognitive maps (Papastamatiou et al. 2011; Mourier & Planes 2011), recognize conspecifics as individuals (Myrberg & Gruber 1974, Guttridge et al. 2011; Jacoby et al. 2014), use the Earth’s magnetic field to navigate (Keller et al. 2021) and to be capable of social learning (Guttridge et al. 2013). Stingrays (Potamotrygon castexi) learned to use water as a tool to extract food (Kuba et al. 2010). Laboratory studies have found elasmobranchs to be capable of a variety of other cognitive tasks including distinguishing between visual objects and electrical fields, categorizing objects, and perceiving illusory contours and bilateral symmetry (Fuss et al. 2014; Schluessel 2015; Brown & Schluessel 2022). Remote technologies of increasing sophistication have been used to monitor shark movements in nature and to infer social networks (Mourier et al. 2019).
Ethology is concerned with the natural behaviour of a species in the environment in which it evolved. It involves close, long-term observation of the behaviour of many individuals to gain knowledge of the species’ behaviour in different contexts. It emphasizes observing animals under natural conditions in order to understand the evolution, adaptation (function), causation, and development of the species’ behavioural repertoire (Tinbergen 1963). Since mental states are closely tied to behaviour (Jamieson & Beckoff 1992), direct knowledge of the actions presented by a species in its natural environment can also provide clues to its use of cognition and other subjective states and how they evolved (Jamieson & Beckoff 1992). To gain such knowledge of a species of elasmobranch, a method for observing their behaviour in detail, long-term, was needed (Gruber & Myrberg 1977).
Carcharhinus melanopterus (Quoy & Gaimard, 1824) is one of the most abundant reef sharks on tropical coral reefs throughout the Indian and Pacific Oceans (Randall & Hoover 1995). Their name derives from the Greek word ‘melas’ meaning ‘black’ and ‘pteron’ meaning ‘fin’ or ‘wing’ Though they are also called ‘blacktips,’ this leads to confusion with the blacktip shark Carcharhinus limbatus (Johnson 1978). Individuals were easy to observe in the lagoons of French Polynesia, where females, juveniles, and the occasional male of the species ranged (Mourier et al. 2011). When casual observation revealed a variety of behaviours not seen in terrestrial wildlife, it was decided to study the community in the local lagoon as individuals. Johnson (1974, 1978), had observed blackfins extensively in Polynesia and had identified Nose-to-Tail following (3.5.1), plus an agonistic display in the closely related grey reef shark (Carcharhinus amblyrhynchos). Yet otherwise, little had been published on the species at the time, with the exception of a collection of anecdotes by Randall and Helfman (1973) in an effort to prove that they are dangerous to humans. More recently, Johann Mourier has studied the same blackfin community, using different methods and focusing on their sociality (e.g. Mourier et al. 2012, Mourier and Planes 2012). However, an ethogram, which is the essential first step in the study of the behaviour of a species (Lorenz 1973) has not been done. The 35 behaviours presented by C. melanopterus individuals, in a variety of contexts over a span of nearly seven years, are described here.
2. Method
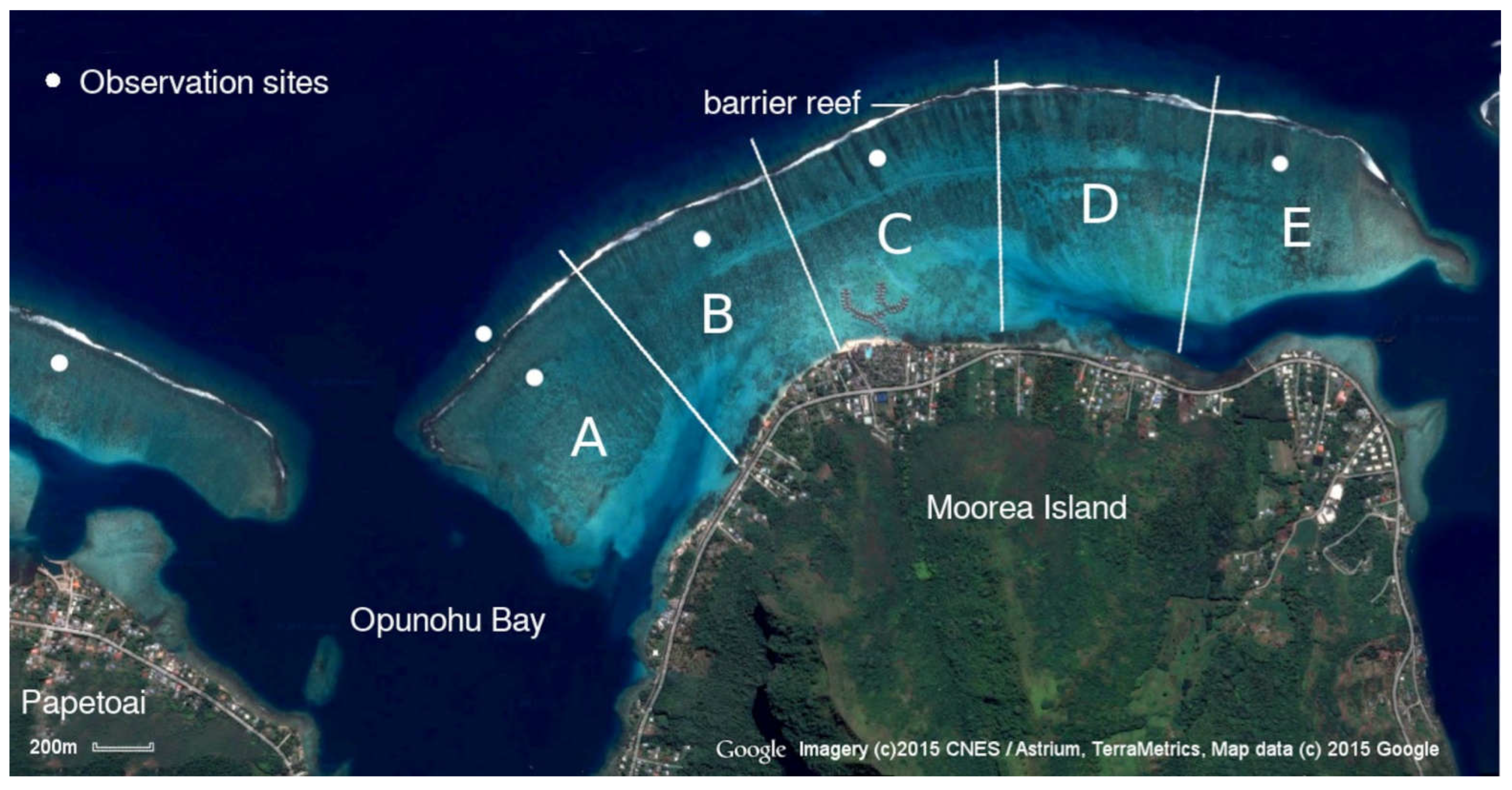
The study area was the lagoon between Opunohu and Cook’s Bay, on the north shore of Mo’orea Island (Galzin and Pointer 1985). It is between 3 to 4 km long, and from 0.8 to 1.2 km from shore to barrier reef. At that time it was a rich coral habitat, about two metres deep on average. It was divided into 5 regions for study purposes (
Figure 1).
Being watched had a marked effect on the wild sharks, so it was necessary to habituate them to the author’s presence. Staged encounters, which involve deliberately attracting the species, provide a method for studying wildlife behaviour (Jamieson & Bekoff 1992). Accordingly, between April 11, 1999 and September 29, 2005, fish scraps were brought once weekly to specific sites in the nearby lagoon. This tactic was effective in habituating the blackfins to the observer, so that in encounters without food between the feeding sessions they appeared to behave naturally. Using provisioning to form a gathering of sharks for observation has since been used by other researchers in Polynesia (e.g. Mourier et al. 2011; Brena et al. 2018) and elsewhere (e.g. Bouveroux et al. 2021).
Feeding sessions were held during the hour before sunset due to the blackfins’ heightened level of alertness at that time. The fish scraps consisted of the remains of oceanic fish after the meat had been removed for sale by a fish shop or hotel, and the amount varied considerably depending on the supply. Since the fishing boats came in on Friday, the scraps were available on Saturday, so the weekly session was held on Saturday at sunset. Occasionally, fish scraps were available during the week, permitting an extra feeding session.
Between feeding sessions, visits were made at random times during the day. The author accessed the lagoon by kayak, went underwater (snorkelling) on the western border, and finned slowly in a zig-zag course eastward for one or two hours, frequently pausing to watch, and moving with any blackfins encountered. Observations were made 1 to 5 times weekly. If the ocean swell exceeded 1.5 m, or the winds exceeded 60km/hr, the session was postponed until the conditions were calm enough. Extra observation sessions were made when some uncommon event occurred, such as a group of rare visitors, a spawning event, a sick or injured shark, a disappearance (the sharks were sometimes absent from the area) or some other unusual circumstance occurred. The lagoon borders, the fore-reef opposite Section A, and the nurseries at both ends of the barrier reef were monitored to acquire supplementary information about the actions of the species.
The sites were chosen in the deeper channel just within the barrier reef, because both older juvenile blackfins and adults were present there, and the patch reefs tended to be large and well-spaced, allowing a fairly unobstructed view through the coral. Each site required an open area of sand to place the food, big enough to allow at least a dozen adult blackfins to circle with ease. There had to be enough space between the patch reefs for them to access the site easily if they followed the scent-trail up-current, and plenty of space for them to exit on the opposite side. A dead coral structure was required, to use for stabilization while watching, up-current from the food. Once located, the same site was always used in each section. Each feeding session followed the same procedure. The anchor was dropped to allow the kayak to line up between wind and current behind the dead coral used for stabilization. Underwater, equipped with mask, snorkel and fins, the author trailed the kayak to the centre of the site, placed the fish scraps on the sand, finned to the dead coral structure, and watched the sharks while taking notes on a slate. The region near the reef was seldom visited by other people so observations could be obtained without dis turbance.
Each blackfin individual encountered was identified using photo-identification techniques (Porcher 2005) complemented with accurate drawings of both sides of the dorsal fin. The length, colour, sex, scars, marks, behaviour, and any other distinguishing features were included in the description of each shark. Over the years, 474 individuals were identified and noted on subsequent sightings (see supplementary material), along with the time, marine conditions, and incidental details. The notes made underwater were entered in a computer spreadsheet on landing and used to write a full description of the session. The region near the reef was seldom visited by other people so observations could be obtained without dis turbance.
Table 1 summarizes the study effort.
3. Results
The blackfins attending the first feeding sessions held in Section A were mature females whose core areas included the feeding site. Here they are termed ‘residents’ of that area. At first they would not eat when the author was present, circling out of visual range, most of the time, with only brief approaches into view. (The visual range referred to is the distance at which the water becomes opaque due to the accumulation of suspended particles). Occasionally one snatched a scrap and accelerated away with it, but it was not until the fifth session that the three mature female residents of that region first approached in
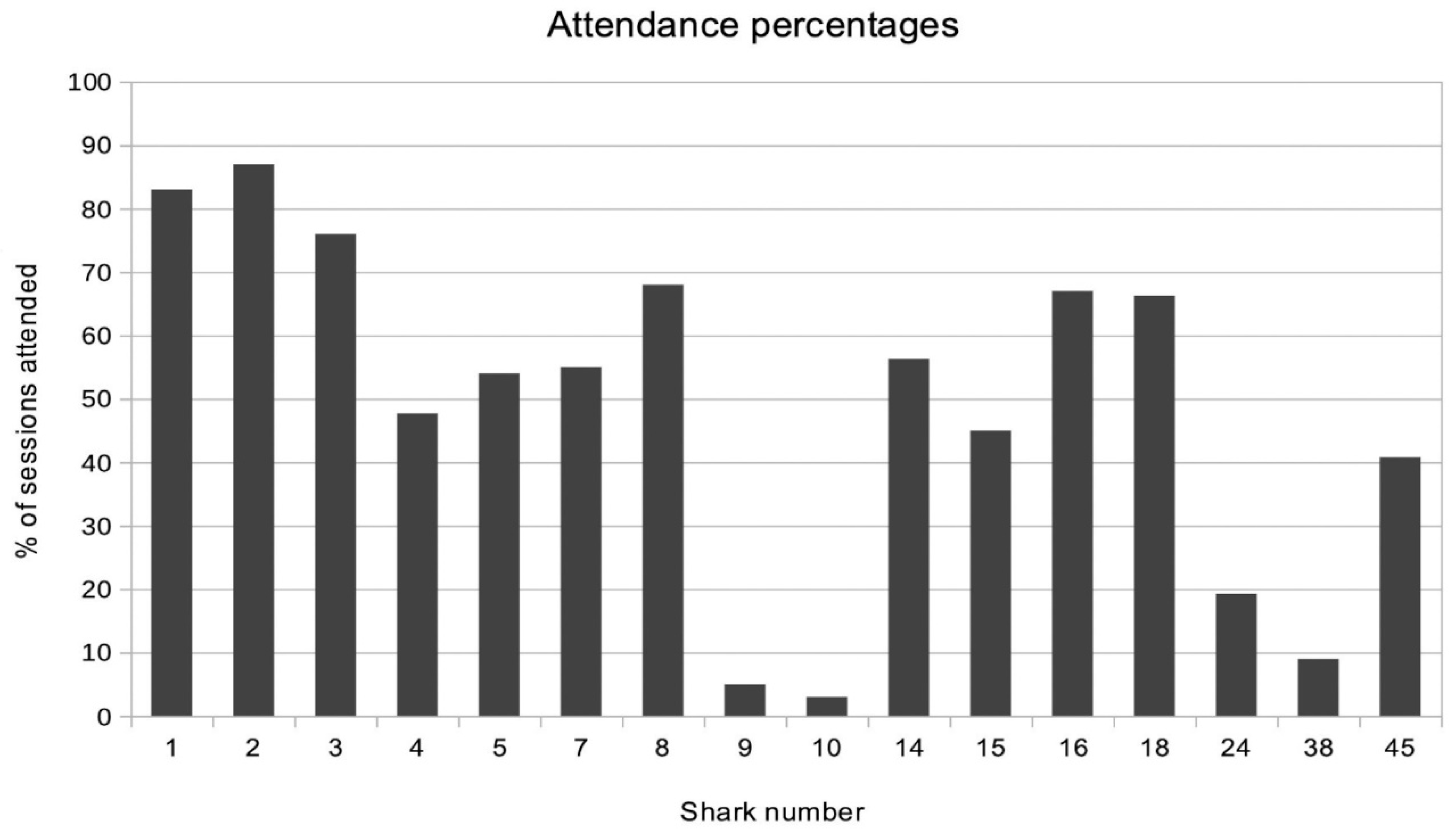
Triangular-Formation (see section 3.5.6), circled the author, then the kayak, approached the food, and eventually chose a scrap. On subsequent sessions, these females, Sharks Nos. 1, 2, and 3, who were soon joined by 8 (see supplementary material), were usually to be found within visual range on arrival underwater. By the time three months had passed, all the blackfins arriving within the first half hour at nearly every session had been identified. Most of these were residents with core areas in the vicinity (
Figure 2).
Blackfins were considered to be residents when they attended more than 50% of the sessions on average, despite being absent, sometimes for extended periods of time, mostly during the reproductive season (Porcher 2005). Residents were also seen regularly in the area on transects without food between feeding sessions. Nos. 9 and 10 were seen a few times over a period of a few weeks and never again. Nos. 4, 5, and 7 were among the few males who resided in the lagoon instead of on the outer slope of the barrier reef. They attended regularly if less frequently.
That first aggregation of sharks would disperse within half an hour, after which as yet unidentified sharks would often arrive. Sometimes they were solitary, other times they came in groups of up to six blackfins of the same sex and approximate size. Each individual was then identified and subsequent sightings were noted. With time and further investigation, it was possible in some cases to learn the locations of these individuals’ core areas. For example Sharks no. 24 and 45 (
Figure 2) were later found to be residents of Section B. In other cases, it was not possible to learn the origin of the visitors, some of which are now known to have come from other islands (Mourier & Planes 2013). Some of the identified individuals only returned annually, often with the same companion(s), apparently in correlation with the reproductive season.
Once habituated to the routine of the sessions, the blackfins circled the region while the scraps were placed, then approached individually to glide above the food. Eventually, one would seize a scrap. If it was a vertebral column, the shark would accelerate away with it, shaking it to remove a piece, with several others in pursuit. When it fell, it was swept up by another blackfin, and energetically shaken again. Thus, it was passed from one shark to another until it was gone or dropped in the coral. The smaller scraps were unhurriedly taken and eaten.
In spite of the presence of food, individuals did not avoid each other, and often touched as they came close to each other while investigating the fish scraps. Video 1 shows 15 minutes of feeding session No. A304, starting at T=9 minutes. The smooth locomotion, velocity, and directional changes typical of the species are seen in the Section A site and vicinity. Details are shown in subtitles (Porcher 2022).
During the six and a half years of this study, C. melanopterus individuals, especially the residents of Section A, were observed in many different circumstances and the following 35 actions were seen as contextual responses to specific situations.
3.1. Locomotion
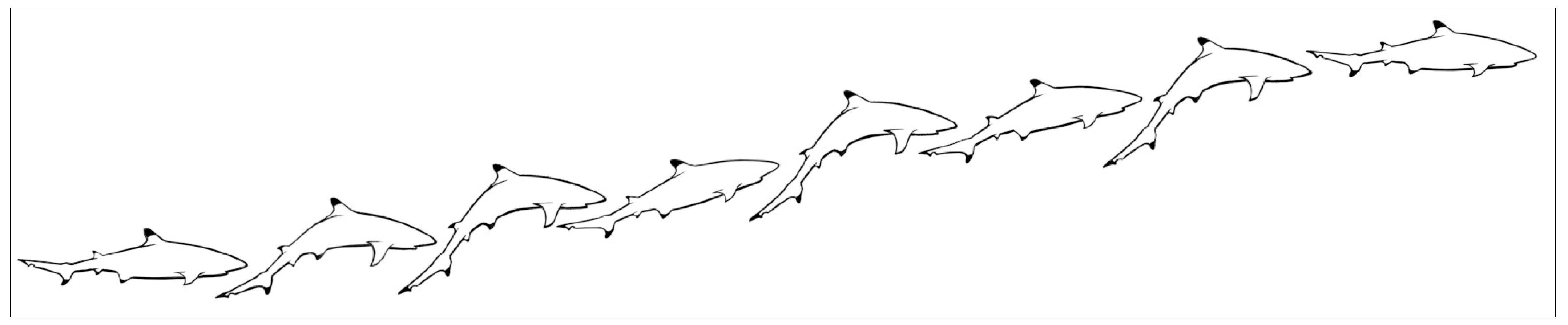
3.1.1 Normal Locomotion
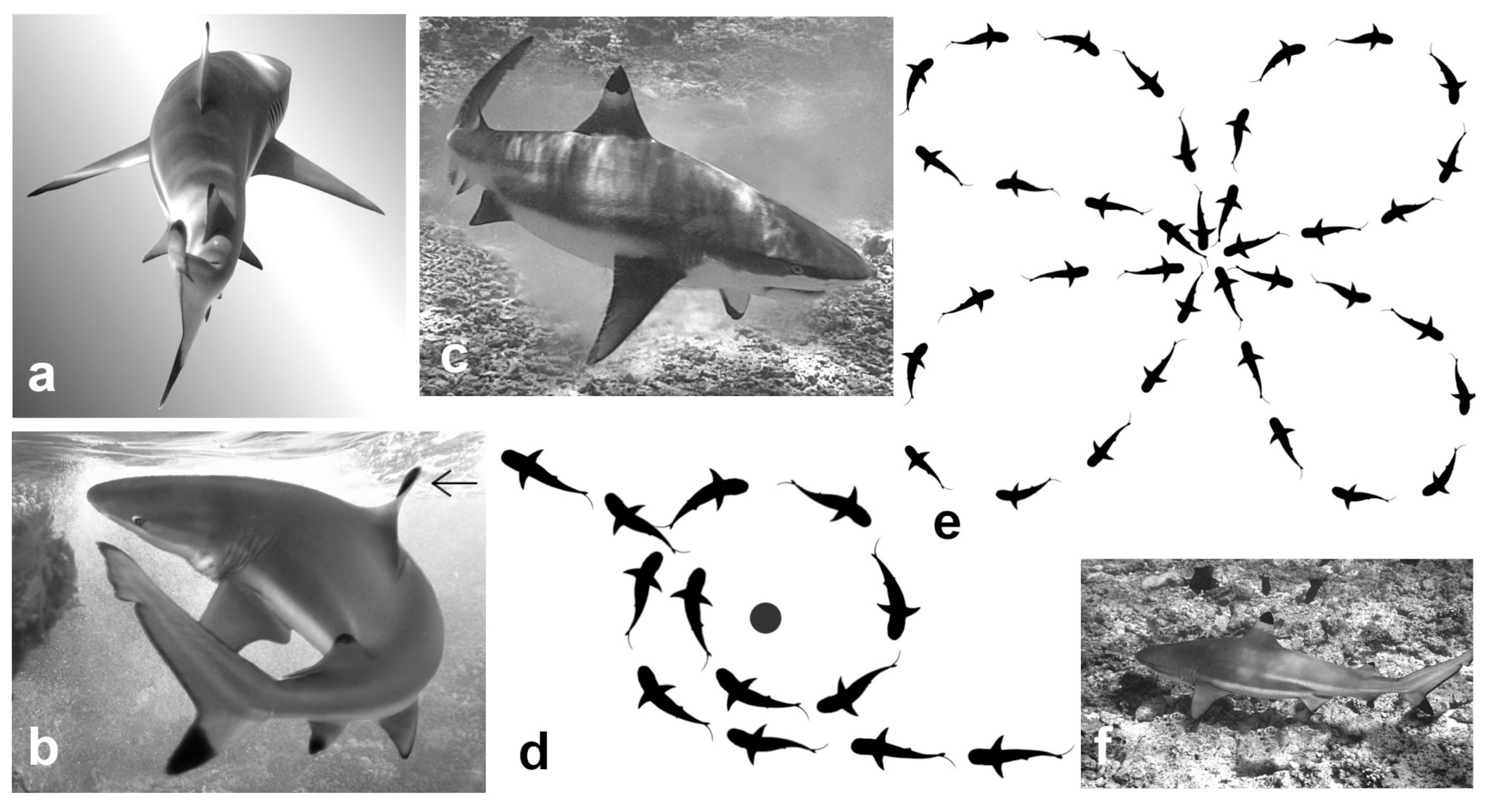
Normal Locomotion is the species’ way of moving forward. The caudal fin beats back and forth horizontally, driving the shark ahead, which results in an undulation moving through the body (
Figure 3a). The shark turns its head in its intended direction, while its fins stabilize it. Video 1 (Porcher 2022a) illustrates
Normal Locomotion by blackfins, which motion is obligatory due to ram ventilation (Jacoby et al. 2015).
Normal Locomotion can be done so slowly that only the tip of the caudal fin moves slightly, or at high velocity, involving strong undulatory motion of the whole animal, while the head remains most stable.
In the shallow lagoon, the blackfin travels in mid-water, from which position it ascends or descends on detecting something interesting enough to investigate.
3.1.2 Braking
The blackfin decelerates by changing its trajectory. Its dorsal fin is seen to be perpendicular to its former direction of travel (
Figure 3b).
3.1.3 Gliding
Gliding intersperses Normal Locomotion. The blackfin’s horizontal undulations almost cease as it moves forward under its own momentum, its pectoral fins in the normal, essentially horizontal position. Gliding results in deceleration, and often occurs when something has apparently caught the animal’s attention and it pauses before possibly altering its trajectory.
3.1.4 Explosive Glide
In an aroused state, the shark accelerates suddenly, then glides for several metres, often arcing around an object of interest. The pectoral fins are usually lowered during this action (
Figure 3c).
3.1.5 Circling
The blackfin circles something of interest. Since it cannot cease
Normal Locomotion to look at something due to obligate ram ventilation,
Circling is the way it stays in one location (
Figure 3d). It begins by fixing its closest eye upon the object, and in looking, it turns in that direction and thus pursues a circular path, usually at a distance of 2 to 3 m. However, at times, certain sharks would circle steadily around the feeding site at a distance of about 15 m, so the radius of the circle appears to depend on the shark, the situation, and the shark’s reaction to the situation.
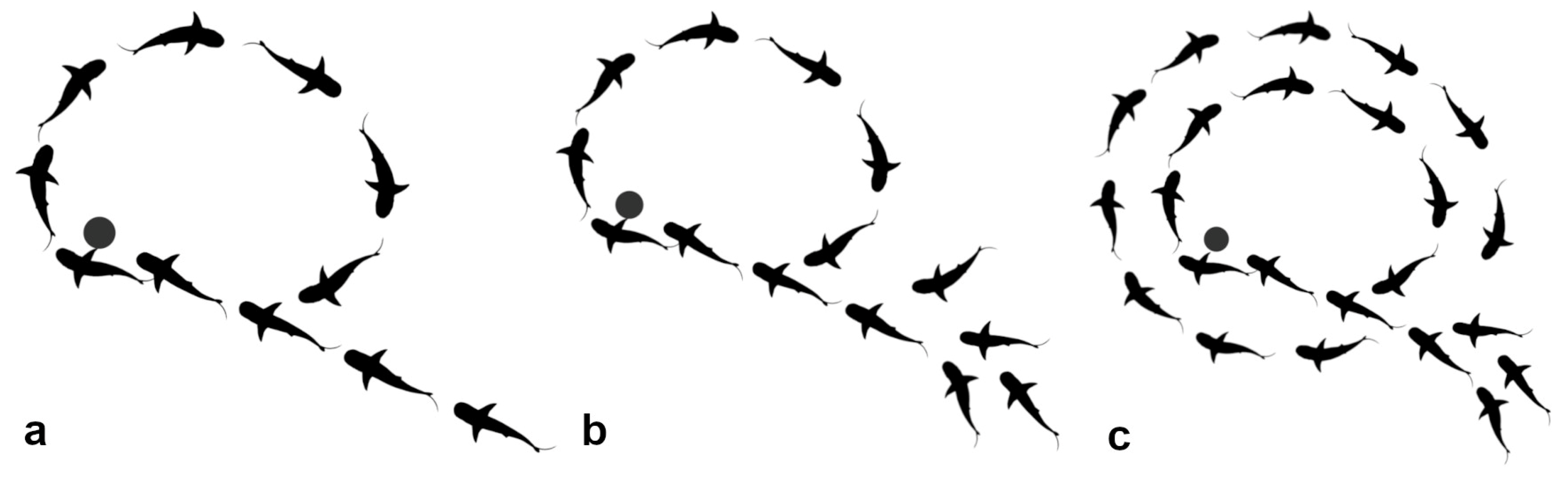
3.1.6 Ranging
When travelling to a specific place or region, the blackfin shark takes a direct route. However, the species was observed to spend a large proportion of its active time following a sinuous path through its preferred range in roughly oval pathways (using
Normal Locomotion). Each new loop is oriented in a different or opposite direction from the last one so that the pathway forms rough figure-of-eights and cloverleafs as viewed from above (
Figure 3e).
3.1.7 Resting
The diurnal rhythm of the blackfins was such that the peak of activity was during the period of low light around sunrise and sunset, when the sun was at an angle of less than 45°. As the sun rose higher they went to a place where the lagoon was relatively open and free of obstacles to cruise slowly,
Resting, a few centimetres above the sea floor, mostly with one or more companions (
Figure 3f). They were much less alert at such times, to the degree that they could be surprised. Their level of activity during the night could not be observed.
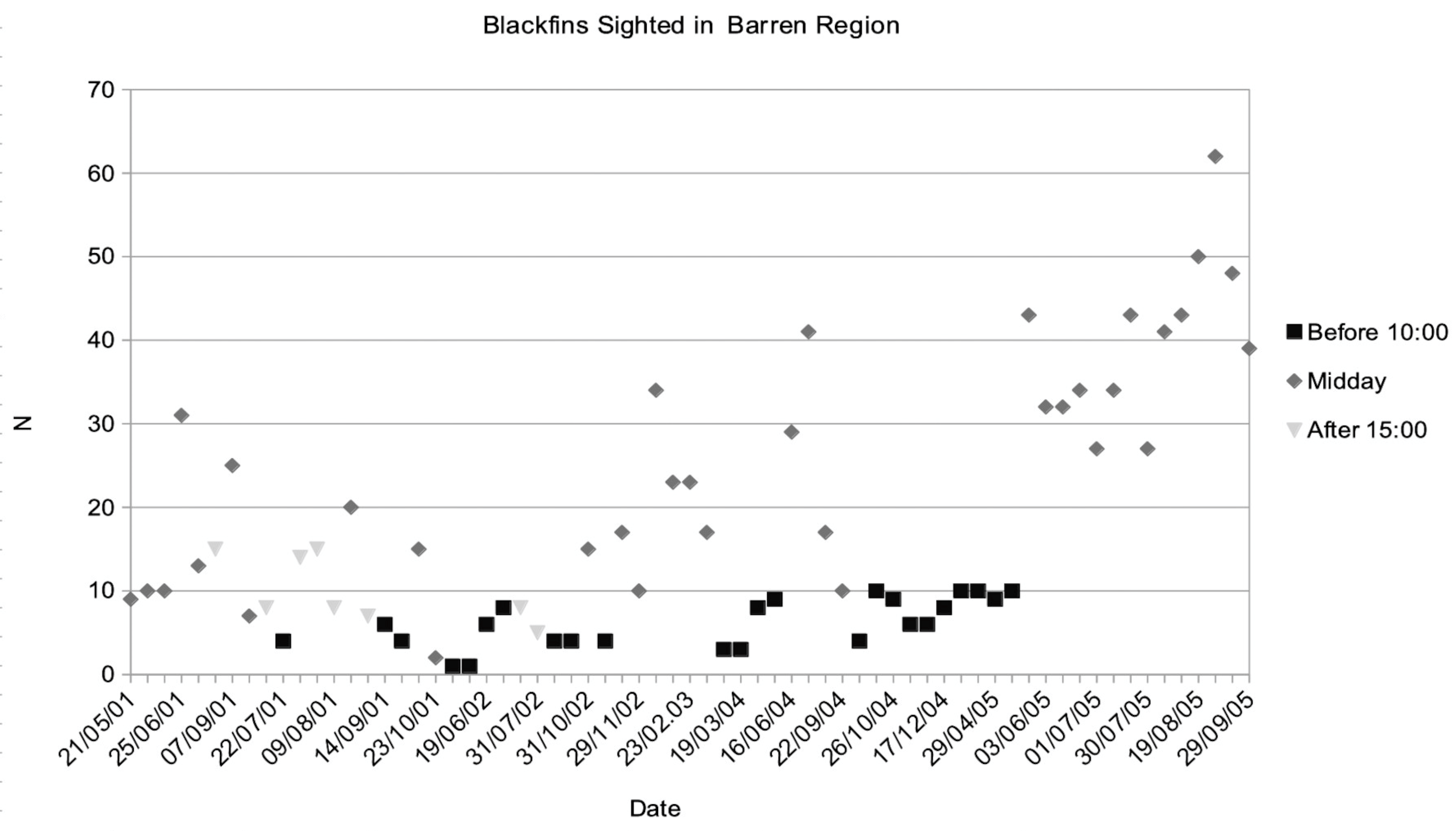
A wide barren region of coral rubble between Sections A and B was deeper than average and used for
Resting by individuals from both Sections.
Figure 4 shows the numbers of blackfins observed there during observation sessions at different times of the day.
At times, some of the females went to deeper water off the fore-reef to cruise, Resting, in mid-water, not far past the turbulence of the waves breaking on the barrier reef. They reportedly did not attend the shark feedings held for divers farther out (Philippe Molle, Boumediene Boucif pers. comm.), which took place at a depth of about 30 m. This suggests that when the females went onto the fore-reef, they remained in relatively shallow water. The blackfins became active again in the late afternoon, after the sun descended past approximately 45° above the horizon.
3.1.8 Breaching
Blackfin sharks
Breach by speeding near-vertically through the surface from below. In the study lagoon, the water averaged about 2 m deep and they began their upward acceleration by turning just above the sand (
Figure 5).
3.3 Grooming Behaviours
3.3.1 Chafe
A blackfin will flip onto its back to wriggle with its dorsal surface against the sand, or whip its side against a sand bank, presumably to free itself of ectopic parasites. It will also position itself to
Chafe its ventral surface on worn, dead coral (
Figure 6).
3.4 Investigative Behaviour
A state of vigilance generally seemed to characterize the actions of the blackfins, yet they showed a strong tendency to investigate things in their environment. This combination generated a variety of tactics that they appeared to use to remain hidden while investigating novel phenomena.
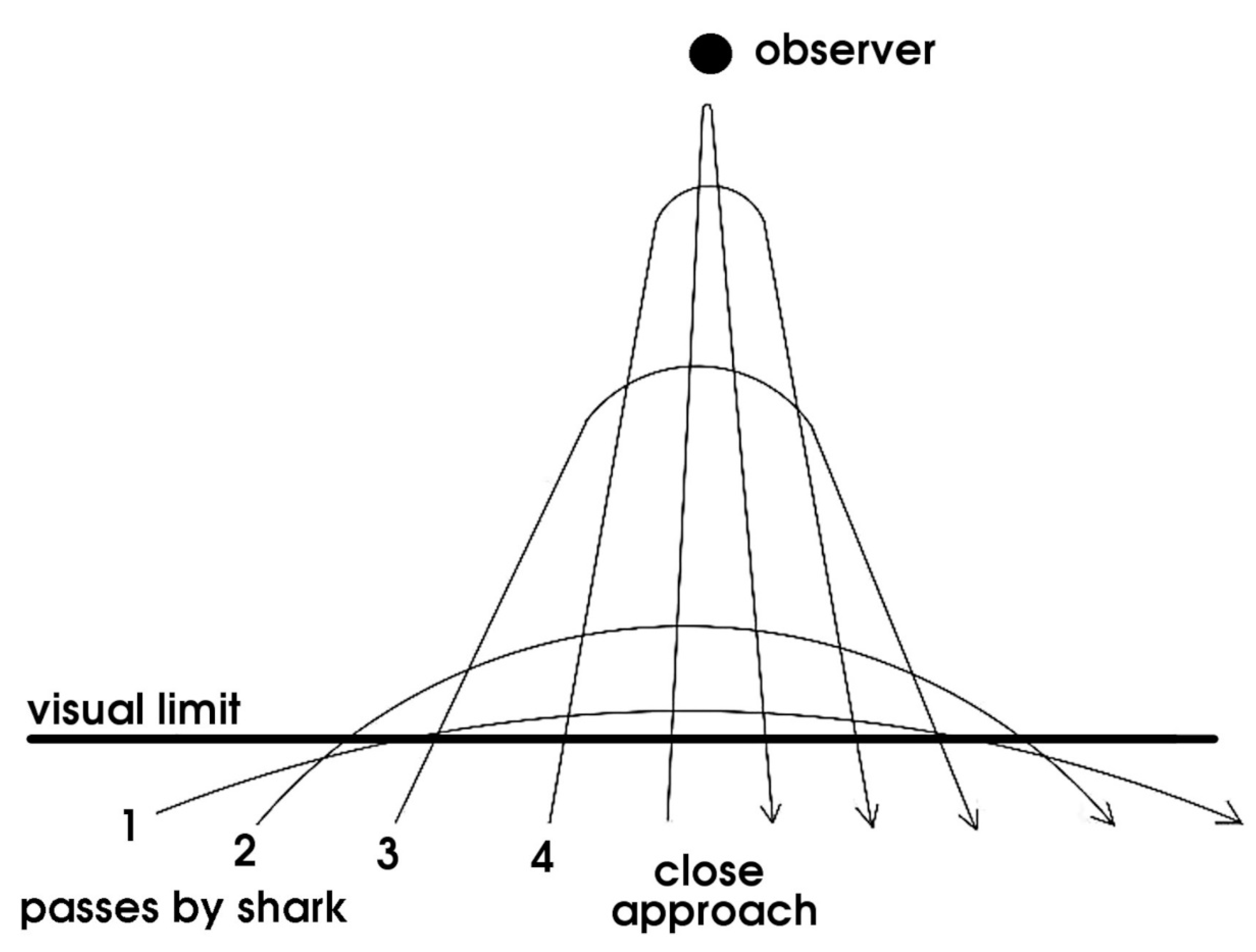
3.4.1 The Approach
At the limit of the observer’s visual range, the shark passes into view and out again. A few minutes later it makes a closer pass. It repeats this pattern, approaching more directly each time, until it might make a direct,
Close Approach (
Figure 7). This general pattern varied depending on the context and individual.
3.4.2 Following
The shark Follows another animal (the observer) beyond visual range. This was exemplified on observation excursions through the lagoon. Certain residents would Follow the author, sometimes for hours, apparently remaining hidden beyond visual range except for the occasional pass into view, usually about once or twice per hour, depending on the individual. It was possible to see which blackfins were Following by waiting, unmoving, until they came into view, usually together.
3.5. Social Behaviours
It appeared that social behaviour was common for the blackfins. Visiting blackfins regularly appeared, singly or in groups of up to six of the same sex and approximate size. The resident females would merge with the visiting females, accelerate, and soar through the vicinity in a ragged linear formation. There were occasions when fish scraps were ignored, suggesting that socializing took precedence over eating at these times. Certain annual visitors arrived year after year with the same companions, though other visitors always appeared alone. Certain groups, including some of those that visited rarely, and certain visitors, aroused more interest and triggered longer periods of higher velocity interaction, than others. During the reproductive season, males frequently arrived at the lagoon sites from the fore-reef as night fell, also in groups of up to six individuals, presumably to seek an opportunity to mate. On occasions when they paused at the feeding site, some of the resident females reappeared and moved with them.
3.5.1 Nose-to-Tail Following
One shark follows another with its snout within 30 cm of the leader’s tail. Resident female blackfins interacting with female visitors were observed to catch up with another female until her nose was at the other’s caudal fin (
Figure 8a). She would usually advance to swim at the other’s side at a distance of about 0.5 m for several seconds before resuming her arcing pathway, then catching up with a different female, and briefly accompanying her before circling on.
3.5.2 Circling Head-to-Tail
Two blackfins follow each other Nose-to-Tail, their bodies arced to form a circle. This was often seen in the smaller juveniles, and especially in the nurseries among the pups, but not in adults.
3.5.3 Swim-Bys
When meeting in the lagoon, companion sharks and acquaintances, mostly individuals of the same sex and approximate size, often perform a
Swim-By, as named by Sperone et al. (2010). The blackfins would arc towards each other until they were approaching head on, then pass each other with an inter-animal distance of <10 cm (
Figure 8b, Video 3) (Porcher 2022c).
3.5.4 Parallel
When two blackfin acquaintances meet, they will often approach each other and move parallel to each other (
Parallel) with an inter-animal distance of < 0.5m. for a few seconds (
Figure 8c, Video 3). They also move in
Parallel as described above (3.5.1), after one individual has caught up to another from behind.
3.5.7 Cutting Across
A blackfin travelling with another decelerates and turns at right angles to Cut Across the other’s path.
3.5.7 Travelling with Companions
The blackfin travels with one or more companions. The wide circles they pursue while Ranging take them out of visual range of each other, then back into each others’ vicinity at regular intervals.
There were places in the lagoon where deeper water and a more open terrain permitted the observation of companions moving through a wide area. They alternately travelled in Parallel, Nose-to-Tail, or independently, pursuing a sinuous pathway of loops and figures-of-eight which repeatedly brought them back together. Shark pups were especially easy to observe from above the surface in the shallows and nurseries. Repeatedly they circled together, then arced away to move through the surroundings, always coming together again through their orbits and figures-of-eights. Often they Circled Head-to-Tail. These continuously circling patterns resembled the ranging patterns of movement presented by the adults on a larger scale.
3.5.8 Circling Together
Sometimes the gathering of resident blackfins Circled Together around something of interest. Most circled in the same direction, though some moved counter to that; individuals usually showed a directional preference. The preferred direction when Circling Together was counter-clockwise among the blackfin residents of Section A.
3.6 Interactive Behaviour
These behaviours involve other species (including the observer), but not conspecifics.
3.6.1 The Close Approach
The blackfin approaches the face directly, then passes close by, or turns away at an acute angle (
Figure 7). When the resident blackfins had become familiar with the sessions at the Section A site, on arrival underwater, up to six of the female residents (depending which ones were present) would turn towards the author and one by one perform a
Close Approach very slowly.
The Close Approach was done at different speeds, depending on the circumstances. At the feeding sessions, a blackfin would occasionally make a Close Approach to a Javanese moray eel (Gymnothorax javanicus), when one emerged from its hole and approached the fish scraps. The shark appeared to pause at a distance that depended on the eel’s reaction to the gesture.
Another variation was used on Tahitian spear-fishermen, who reported (various pers. comm.) that blackfins would harass them in an effort to get their catch of fish (they said) by dashing up to their faces and away again.
3.6.3 Charging
Under certain circumstances, the blackfins would
Charge, advancing at medium to high speed and passing just to one side (
Figure 9a).
Charging differed from the
Close Approach in that it was directed towards the mid-body and was usually repeated at intervals. Conspecifics might join in, often in
Follow-Formation.
3.6.4 Circling Charging
On rarer occasions,
Charging, circling away, and
Charging again, results in
Circling Charging as other sharks join in and their level of arousal increases (
Figure 9b). Other blackfins join in
Follow-Formation initially, following the leading shark directly up to the target before turning away. Then these individuals will often begin
Charging independently (
Figure 9c). A period of an hour is the longest that the author witnessed
Circling Charging to continue, and the entire gathering of 36 blackfins, including two rare visitors, took part. This behaviour pattern can break out into
Slamming.
3.6.5 Slamming
The blackfin accelerates towards a target and
Slams into it with the force of its momentum. The shark might
Slam initially with the shoulder (Porcher 2016), and as its level of arousal grows, it approaches from below and spirals upwards with arched back to
Slam with the region behind the head in front of the dorsal fin, lowering the pectoral fins just at the moment before impact (
Figure 10).
Slamming can be done with great force.
These actions were seen in a variety of circumstances. Once a leading shark had begun, the others present joined in. Intimidating, high-speed Close Approaches became Charging, Circling Charging, then Slamming, and involved the entire company of blackfins present (Porcher 2021).
3.6.6 Undulating Against Another
The shark
Undulates Against Another body. During this study, but after several years, certain residents began
Undulating Against the author’s kayak on its arrival in the lagoon. Initially, they
Undulated Against the boat while remaining at the surface (
Figure 11a).
After two episodes, the individuals involved began ascending directly upwards from the sand beneath (
Figure 11b),
Undulating Against the craft (
Figure 11c), then turning to go straight downwards, so that their tails emerged through the surface (
Figure 11d). Several performed this action at once, so that their tails flicked through the surface at the same moment, several on each side of the kayak. This was therefore a different action than simply performing a brief
Undulation Against Another at the surface. These individuals performed this action each time the author arrived at Site A thereafter.
- b)
The blackfin turns at the sand.
- c)
On arrival at the surface it Undulates Against the other.
- d)
The blackfin descends.
3.7. Fear Reactions
3.7.1 Bolting
The shark accelerates out of visual range. This is the usual fear reaction (Video 4) (Porcher 2022d).
3.7.2 Startle Response
The blackfin accelerates away from a source of fear while arching its back vertically and depressing its pectoral fins in a series of rapid jerks. The arching of the back results in the tail pointing downwards, which causes the shark to rise into the open area above the coral as it flees (
Figure 12) out of visual range.
The Startle Response was commonly seen in juveniles as a reaction to a near-collision with a much larger shark, usually a nurse shark (Ginglymostoma cirratum). Adult blackfins too, would sometimes accelerate away with a few sharp vertical undulations when Startled by a near-collision with another shark.
3.7.3 Shiver Mode
After fleeing as a result of the Startle Response, the blackfin is likely to circle back in Shiver Mode. Shivers run through its body, and it suddenly twists and changes direction erratically. Though Shiver Mode was usually seen in juveniles, shivering and unpredictable turns were also occasionally observed in adults who appeared in an aroused state for unknown reasons. Moving swiftly, while shivering and twitching at times, the animal would make sudden rushes, then whip back around in a tight circle. The restless Shivering, flicking, and twisting usually continued for less than one or two minutes, but on one occasion an individual remained in Shiver Mode for about 20 minutes, suggesting that it can also indicate a longer-term negative subjective state (Porcher 2023).
4. Discussion
Watching the individuals in this community of wild blackfins in semi-controlled conditions over a period of several years provided the opportunity to gather much information on their natural behaviour. Many of their actions have been noted by other researchers, some in other species, which suggests that they might also be present in the behavioural repertoire of closely related species, and possibly evolved in ancestral forms.
4.1. Locomotion
The blackfins’ way of moving, here termed Normal Locomotion, was first described by (Myrberg & Gruber 1974), in bonnethead sharks (Sphyrna tiburo), who also documented the Explosive Glide.
The Ranging pattern observed closely resembles the descriptions of Stevens (1984) at Aldebra Atoll (Seychelles). He wrote that the blackfins appeared
“to be continually searching, crossing and recrossing their path, often remaining in a small area for an hour or two before moving off and repeating this pattern in another area. They rarely moved in a straight line for more than a few minutes.”
Papastamatiou et al. (2009b) described blackfins at Palmyra Atoll (Line Islands) using patches that were 3 to 17% of the scale of their home range for periods of days. They repeated straight movements along ledge habitats, which was theorized to be the most efficient search strategy when foraging is unpredictable. They moved using a “direct walk” while in preferred foraging patches, and appeared to move randomly between them.
The observed Ranging pattern provides good coverage of a given region, and appears to be well suited for locating prey visually, as well as intercepting scent flows, including the chemical signatures of conspecifics from beyond visual range (Johnson & Nelson 1978). Blackfins would presumably also be aware of the underwater sounds made by other sharks within range (Myrberg et al. 1976) through hearing and the lateral line sense (Bleckmann & Zelick 2009; Gardiner & Atema 2007, 2014).
Papastamatiou et al. (2015) found that the need to regulate the body temperature had a strong influence on the species’ daily routines in Palmyra Atoll. At different times blackfins chose warmer or cooler water said to serve their needs. They presented a peak of activity in the low-light period at and just after sunset, for at that time, blackfins not only have a sensory advantage over their prey, but also a thermal one (Papastamatiou et al. 2015). They found that the blackfins of Palmyra Atoll tended to Rest in deeper water (Papastamatiou et al. 2015), which correlates with the patterns of Mo’orea blackfins observed.
In the barren area or in the ocean where the blackfins in this study went to Rest, their forward motion was relatively unimpeded by coral structures. Further, in the deeper water, they were out of the brilliantly lit shallows of the white-sand lagoon. Papastamatiou et al. (2009a) found that C. melanopterus in Palmyra Atoll presented a diel pattern similar to that of the blackfins of Mo’orea, slowing their velocity during the day and becoming more active at night. They also noted that multiple sharks Rested in the same area, as was observed here. A possible reason might be due to the blackfins’ lowered level of awareness at these times. With many sharks Resting in the same region, if there was a disturbance, the reaction of the sharks directly affected would be perceived by others through the lateral line, even though the disturbance was beyond visual range.
Myrberg & Gruber (1974) documented bonnethead sharks moving with increasing velocity as the afternoon passed, while Gruber et al. (1988) noted that juvenile lemon sharks apparently used the sun as a visual cue for their diel movements.
The only time that the blackfins Breached in this study was to access the fish scraps carried in the back of the kayak. After they broke through the surface, they saw them, leaned towards them, and snapped at them—they had found a new foraging technique. Breaching to access the scraps was done independently by two different gatherings of sharks. In one case it was initiated by one shark, in the other by three or four, then the others present did the same, apparently by copying them. This appeared to be a case of social learning (Guttridge et al. 2013).
4.2 Eating Behaviour
A series of actions similar to Search Mode was documented in bonnethead sharks and termed Manoeuvring by Myrberg & Gruber (1974), who noted that it had been reported in other species and was thought to be the sharks’ way of orienting to a spot on the substrate. They also described the Head-shake, which they saw not only when feeding, but in association with the Explosive Glide (Myrberg & Gruber 1974) and what they termed ‘competitive situations.’ The Head-shake has also been documented in grey nurse sharks (Smith et al. 2015). Klimley (1985, 2023b) noted that scalloped hammerhead sharks (Sphyrna lewini), perform a Head-shake as a social reaction while retreating after a competitive encounter. However, the blackfins did so only to extract a bite of food from a large piece, with the one exception of the attempt to be rid of a remora (3.2.5).
The way the blackfins took up a scrap, used the Head-shake to saw out a bite and dropping it, then to be caught up by an accompanying conspecific until it was gone, is curious. In this situation, it would appear not to be the best nutritional strategy. While they were thus occupied, often the fish and nurse sharks took the rest of the fish scraps. The blackfins would all have benefited more had each one taken its own piece of food. Since in general blackfins are solitary predators (Frisch et al. 2016), an explanation for their actions is not evident. Possibly they frequently acquire pieces of food that are large enough to eat this way—for example, when feeding off a large carcass. Indeed, this behaviour pattern suggests that blackfins do have a tendency to eat together. Further observations could serve to throw light on this behaviour.
Though C. melanopterus usually took the fish scraps in their jaws, they were observed at times to suck in a scrap from a distance of up to about 20 cm, after which there was a burst of cloudy water from the gills. This resembled the suction feeding described for nurse sharks (Motta et al. 2002) in which the shark expands its buccal cavity and protrudes its jaws, creating a suction that pulls the food in. Then, as the parts return to their normal position, the decreasing volume inside the mouth forces the water out through the gill openings.
Palatoquadrate adjustment was regularly seen in the blackfins during and after feeding, and has also been noted in the grey nurse shark (Carcharias taurus) (Smith et al. 2015). The palatoquadrate is not fused to the brain case (Moss 1972) and the jaws are supported instead by the cartilaginous hyoid elements, which brace them against the cranium when the shark bites down. This arrangement permits the jaws to move independently from the rest of the shark’s head (Moss 1972).
It was repeatedly noted that after an act of predation by a blackfin, conspecifics in the vicinity suddenly went into high-velocity Search Mode. This might be due to the fish releasing what has been termed a “fright” substance that warns conspecifics of danger (von Frisch 1941), or possibly fish vocalize when caught. Otherwise, the cause of this reaction is unknown.
4.3 Grooming Behaviours
Other studies have reported a variety of species of sharks Chafing against the sand (e.g. Myrberg & Gruber 1974; Ritter 2011; Smith et al. 2015; Williams et al. 2021), but here the blackfins were observed to turn on their backs on the sand and rub their ventral surfaces against smoothly-worn dead coral structures. Their consistent choices of an appropriate feature on which to scratch, whip against, or Chafe upon, suggest active structures selection, providing an example of decision making (Wang et al. 2004).
4.4 Investigative Behaviours
The apparently systematic use of the visual limit for concealment by C. melanopterus suggests that individuals of this species are comfortable perceiving events from beyond visual range, apparently by integrating information from other senses. Moving through a vibrating realm in which light travels less easily than sound, blackfins’ use hearing (Myrberg 2001) and the lateral line sense (Bleckmann & Zelick 2009; Gardiner & Atema 2007, 2014), which directly perceives underwater vibrations. Sharks in general hear well (Myrberg 2001) and are particularly sensitive to low frequency sounds of 40 Hz to approximately 800 Hz, which includes sounds made by other animals moving in the vicinity. The lateral line sense does not respond to acoustic stimuli (Myrberg 2001), but to pressure changes, such as those generated by other animals as they move, and to waves made by their own actions, bouncing off obstacles (Bleckmann & Zelick 2009; Gardiner & Atema 2007, 2014). It even contributes to the location of scent flows (Gardiner & Atema 2007). While the ampullae of Lorenzini are extremely sensitive to electric stimuli, heat, mechanical influences, and salinity (Kalmijn 1966; Josberger et al. 2016; Anderson et al. 2017; Sisneros & Tricas 2002), they work at close range only.
Shark vision has also been found to be acute (Gruber & Cohen 1978), and the blackfins’ repeated passage into visual range suggests that visual input was also important to them. Myrberg (1991) hypothesized that contrasting fin markings may play a role in species recognition in C. melanopterus and sympatric reef sharks, which emphasizes the importance of vision in these animals that spend much of their lives in clear and shallow waters studded with coral obstacles.
The blackfins’ pattern of Following another animal while remaining beyond visual range is another example of their routine integration of input from several senses to analyse ongoing events. They doubtless also Follow other species, remaining beyond visual range.
During the blackfin sessions it was noted that the whitetip reef sharks (Triaenodon obesus) and sicklefin lemon sharks (Negaprion acutidens) attending intermittently also used the visual limit for concealment. Tiger sharks (Galeocerdo cuvier) and Atlantic lemon sharks (Negaprion brevirostris), observed for shorter periods of time in the Bahamas, performed in a similar way. When approaching a gathering of scuba divers and other sharks, they passed just within visual range for a look, came closer < 5 min later, then nearer again after another delay, though it was observed that tigers tended to pass overhead for a close look. For oceanic species especially, the visual limit is the only thing available to use for concealment, which might explain why it is used by so many species.
4.5 Social Behaviours
Nose-to-Tail following in C. melanopterus was first documented by Johnson & Nelson (1978); a male blackfin approached a female from behind and followed Nose-to-Tail prior to copulation. Mourier et al. (2011) later noted blackfins following Nose-to-Tail. This behaviour has also been reported in bonnethead sharks (Myrberg & Gruber 1974), basking sharks (Cetorhinus maximus) (Sims et al. 2000), white sharks (Carcharodon carcharias) (Martin 2003), and oceanic whitetip sharks (Carcharhinus longimanus) (Gallagher et al. 2014). In both white sharks (Martin 2003) and bonnetheads (Myrberg & Gruber 1974), it was observed that Nose-to-Tail following occurred most often with the arrival of a newcomer. That is consistent with these present observations of socializing blackfins approaching each other from behind and following briefly Nose-to-Tail when visitors were present. Circling Head-to-Tail, in which both sharks follow Nose-to-Tail simultaneously, was reported in bonnethead sharks by Myrberg & Gruber (1974).
Moving in Parallel was noted in this same community of blackfins by Mourier et al. (2011), and in white sharks by Sperone et al. (2010). They also documented Swim-Bys being performed by white sharks and specified that this social action, along with moving in Parallel, was most often performed by individuals of the same sex and approximate size, as was seen here among the blackfins.
Follow-Formation was described in bonnethead sharks by Myrberg & Gruber (1974) when 3 to 6 sharks followed another in single file. Though noted rarely, it happened 12 times after the biggest female was introduced. However, in that case the Followers consisted of males, while here, Follow-Formation was most often presented by socializing female blackfins (within the lagoon).
As in the bonnethead sharks, Follow-Formation formed most often with the appearance of certain individuals. For example, when Shark No. 24, an aged female from Section B who was also one of the biggest blackfins attending, visited Section A, she always attracted attention from those present and Follow-formations formed and dissolved behind her almost continuously as she moved. On one occasion, she performed a U-turn in a narrow coral canyon, and all six of her followers did too.
The natural sex segregation in the wild blackfins as opposed to the bonnethead sharks’ being in a state of confinement in an aquarium, could explain this difference. Possibly the bonnethead sharks were not stimulated to take Follow-Formation by the type of unexpected events that regularly confronted the wild blackfins. As Myrberg & Gruber (1974) noted, these formations are context-specific.
Follow-Formation was not only adopted by the blackfins when following a conspecific, but also when confronted with a novel scene. An example is the blackfins’ reaction to perceiving a scuba diver (who once accompanied the author to a session) for the first time. After disappearing beyond visual range for a full 10 min. they suddenly came forward in Follow-Formation: 35 blackfins in two lines, single-file, following two sharks who had shown leadership tendencies in the past (Guttridge et al. 2011). Along with adopting Triangular-Formation in challenging situations, this type of response is a possible indication of social buffering in the species (Kikusui et al. 2006), wherein individuals prefer to approach novel situations or objects together rather than alone. Further observation of this type of behaviour in blackfins and other species might provide greater understanding.
Triangular-Formation was seen to be adopted more rarely, when individuals confronted challenging situations. The best example of it observed in this study was the initial approach to a feeding session by Sharks Nos. 1, 2, and 3—Session 5 on 1999-05-07. They came in Triangular-Formation. Though No. 3 was the individual who usually took the lead among those residents, in this case, it was No. 2, who otherwise did not distinguish herself, who was in the leading position. Three years later—at Session 247 on 2002-05-11—those same three sharks, in the same positions in Triangular-Formation, left the centre of the gathering where the food was and accelerated forward to Slam the author. After being fought off, they immediately regained the same positions in Triangular-Formation to return to the food area. Such instantaneous, seemingly organized behaviour presented the appearance of planning and structure, yet the blackfins’ movements were always flexible and liquid—the formations suddenly formed and dissolved with no visible sign of any communication taking place. The implications of this type of behaviour, in terms of the social nature and cognitive capacity of wild blackfins, would likely be a rewarding subject for future field research.
The circling Ranging pattern used by blackfins is evidently suited to crossing and recrossing scent trails as well as visible prey. Given that we know that this species picks up the scent trail of conspecifics (Johnson & Nelson 1978), we can hypothesize that blackfin companions remain in loose contact through repeated crossing of each others’ scent trails. While it is also likely that they are aware of conspecifics in the vicinity using hearing and the lateral line, the way they would systematically follow companions and other conspecifics to catch up with them from behind, suggests that chemoreception is likely the primary focus in this context.
Cutting across was used flexibly in a variety of circumstances and differently by different individuals. For example, Shark No. 1 always did it if the author tried to accompany her, prior to accelerating away. On one occasion an accompanying blackfin Cut across when the author was carrying fish scraps. On several occasions a blackfin who had been travelling in the vicinity of the author for a period Cut Across before turning 180° and departing. An unknown female blackfin did it 5 times while being followed 1.5 km along a different lagoon for 35 minutes.
When the Section A blackfins Circled Together, the direction was counter-clockwise although two or three would circle clockwise, counter to all the others. Most of the blackfins appeared to have a preferred direction, though it was not evident whether they had a preferred eye or a preferred circling direction, or both. Lateralization has been widely found across the animal kingdom (Brown 2014) and closely analysed in fish (Brown 2014; Bisazza and Brown 2011). However, the pattern varies between species, populations and individuals, so the presence of lateralisation in the blackfins is impossible to interpret with relevance here.
4.6 Interactive Behaviour and Agonistic Displays
Agonistic displays have been seen in a variety of shark species. The first was documented in the grey reef shark by Johnson & Nelson (1973). When grey reef sharks were chased and cornered, they would arch the back, raise the snout, lower the pectoral fins, and swim to wards the offending diver with exaggerated horizontal undulations, sometimes rolling or looping in a spiral. Then they would either flee or, with a lightning gesture, deliver an open -mouthed, slashing attack. The display increased in intensity depending on with the speed and directness of the diver’s approach, and the degree to which the escape route was blocked.
Since then, more agonistic displays have been identified. Klimley et al. (1996) found evidence of a ritualization of conflict in the great white shark. When a seal that one of them has killed comes under dispute, each slaps the water at an angle with its tail, and the shark that raises the most water and blasts it farthest, wins the prey (Klimley et al. 1996; Klimley 2023a). The exchange appears to be a communication involving a show of power, which allows the great sharks to decide and agree on which of them will get the seal. Thus a physical battle that would badly damage both sharks is avoided.
Martin’s (2007) review describes agonistic displays in 23 species of sharks from 6 families including the great white, the tiger (Galeocerdo cuvier), the sand tiger (Carcharias taurus), the scalloped hammerhead (Sphyrna lewini), the silky shark, (Carcharhinus falciformis), the blue (Prionace glauca), several reef sharks, and the basking shark (Cetorhinus maximus). Jerky, exaggerated movements or sudden turns, accompanied by the sustained depression of the pectoral fins, are most typical. The shark might also arch its back, hold its mouth open, billow its gills, or gape repeatedly. Smith et al. (2015) observed Charging in grey nurse sharks (Carcharias taurus). On the Great Barrier Reef, silvertip sharks (Carcharhinus albimarginatus) performed a stiff-bodied display towards divers. After an initial acceleration away, several returned to a distance of about two body lengths, turned broadside, and moved slowly past the divers. Each displaying shark lowered its pectoral fins and tail, gaped its jaws rhythmically, and vibrated its entire body as if shivering, while passing. Then it accelerated away (Martin 2007). The sandbar shark (Carcharhinus plumbeus) has also turned broadside to divers with depressed pectoral fins. Female sandbar sharks were reported to have rammed divers with their snouts, while the males veered off without coming closer than a body length away (Martin 2007).
Martin’s comprehensive review (2007) indicates that agonistic displays are likely widespread in elasmobranchs, but what evidence exists is mostly anecdotal and incomplete. Though the grey reef shark’s posturing is exceptional in its form and presentation, in other species such displays seem less clearly defined or predictable (Martin 2007). As in the blackfins, they are likely used flexibly according to the circumstances, which makes them challenging to describe in structured terms, particularly when they have only been observed incidentally and not in a variety of circumstances.
When the Close Approach is done fast, it appears to carry the intention of intimidation, as exemplified by the way blackfin sharks accelerate up to spear fishermen, with the apparent intention of acquiring their catch. In such cases, it falls under the definition of an agonistic display, though lacking the components of vertical hunching and muscular flexing. However, these are present in Slamming behaviour, into which the Close Approach can escal ate. But Slamming is not a display. It is an instantaneous attack without pre -signalling (Porcher 2016).
Other researchers have concluded that such actions by sharks are intentional efforts to communicate a negative subjective state (Johnson & Nelson 1973; Klimley et al. 1996, Martin 2007), and several of the blackfins’ actions, especially Charging, Circling Charging, and Slamming, also presented as such (Porcher 2021). The incident in which the blackfin gathering accelerated from beyond visual range up to the face of the visiting scuba diver in 2 lines of Follow-Formation (4.5) appeared to be a mass Close Approach done fast. The diver was enveloped in a swirl of swiftly moving sharks and he fell over backwards. The sharks’ action appeared to be an attempt to intimidate, and the diver, though an experienced dive club owner and shark diver, was indeed intimidated.
In the case of blackfins approaching Javanese moray eels, the Close Approach resembled predator inspection, with the inspector attuned to the reaction of the inspectee. Predator inspection is a behaviour pattern that has been described in certain fish and other taxa (Fishman et al. 1999). It involves members of a prey species approaching a predator, apparently for a closer look. Here, the Close Approach by a blackfin to an eel involved another meso-predator in a situation involving competition over food rather than fear of being eaten. Only juvenile males were observed to approach the big eels in this way, suggesting that the older blackfins might have learned something about them from experience. Johnson (1978) includes an incident wherein he witnessed a Javanese moray eel wrap itself around a shark’s gills after a dispute over food, whereupon the shark tried to bash the eel off by writhing and whacking itself on the surrounding coral structures (Johnson 1978).
Martin’s review (2007) names C. melanopterus among the species that will display, yet includes no details, mentioning only that blackfins will lower their pectoral fins while turning past a diver. However, this observation seems inconclusive when they lower their fins in tight turns (3.2.1). In spite of attending recreational dives with dive clubs on several islands as well as Mo’orea, in which blackfin sharks were among those present, the author did not identify any such agonistic gesture towards divers.
Martin does not identify the commonly-presented Close Approach of the species as an agonistic display, nor the Startle Response. This could be due to his method of pursuing the sharks, but not startling them. Nelson et al. (1986) used a diver propulsion vehicle to trigger agonistic displays in various species of sharks through “sustained, oriented pursuit” and stated that they had been unable to illicit an agonistic display in C. melanopterus.
The blackfins’ Startle Response presents as being homologous to the grey reef’s agonistic display, but it is neither a display, nor agonistic. It appears to be a reflexive reaction that evolved to protect the shark by making it harder to grab by a predator. The swift lowering of the pectoral fins at the moment the shark is startled could be associated with its rise in the water column as it flees the area. An ascent from the more crowded region on the reef into the open space above the coral structures is typical of responses in which a blackfin rapidly departs, and this upward trajectory is also achieved by the arching of the back, resulting in the powerful tail pointing downwards. The vertical jerking during acceleration as the blackfin takes flight likely enhances predator avoidance by making the shark more difficult to grasp.
Myrberg and Gruber (1974) identified a startle reaction in bonnethead sharks, however in their case, no vertical jerking was noted. They also defined a Hunch posture which caused the tail to lower. However, this was not connected with fleeing, but was described as an agonistic display towards a newcomer in the pool, a smaller shark, or a diver present.
Carcharhinus melanopterus arched the back and accelerated upwards while Slamming, but never in warning. By the time the back hunched, the shark was already launching itself, and it lowered its pectoral fins only momentarily, less than a second prior to impact (Porcher 2016).
Slamming behaviour has not been reported elsewhere, and how it normally expresses is unknown. Possibly blackfins Slam large floating carcasses together, in order to break them up and help free pieces of food. However, on no occasion did any of the sharks Slam into their pile of fish scraps to break them up and make them easier to access. During this study, including two occasions on which blackfins suddenly Slammed the author personally, it was used only as a method of attack (Porcher 2016, 2021).
Although Martin (2007) mentioned a brief “shivering” in the silvertip shark during an agonistic display, his description does not resemble the shuddering and randomly darting behaviour, here termed Shiver Mode, that often followed the Startle Response in the blackfins, and which left no doubt that the behaviour was part of a fear reaction. Tester & Kato (1966) noted in their experiment on visual discrimination in C. melanopterus that when one was given an electric shock, its body “twitched noticeably,” which helps to confirm that blackfins appearing in Shiver Mode, which also involved repeated twitching, had been negatively disturbed (Porcher 2023).
It is possible that gestures and displays in the various species of sharks differ between geo graphical regions. The blackfins of the volcanic islands in the central Pacific are descended from those individuals who crossed thousands of kilometres of ocean from the continents to the west to find them (Maisano Delser et al. 2019). Their repertoire of behaviour patterns could have changed as they crossed the Pacific Ocean, and were subject to different conditions and exper iences while remaining isolated. For example, Martin (2007) found that, unlike the grey reef sharks of French Polynesia, those in the waters off Queensland would not display: when chased, they simply departed.
A curious aspect of the blackfins’ behaviour is the way certain individuals would begin an action, and, at times instantaneously, be followed by the rest of the blackfins present. This was especially notable in Charging and Slamming. When aroused, they moved so fast and decisively that they appeared to act in unison, though in many cases the author had seen the leaders and knew the context (Porcher 2021). The behaviours were initiated by one or a few blackfins and picked up by the entire gathering. This tendency suggests the possibility of emotional contagion (Hatfield et al. 1993), in which subjective states are communicated among other conspecifics present. Further investigation would be necessary to confirm this as a facet of shark social behaviour.
The lack of intra-specific aggression documented among the blackfins in this study, even in the presence of food, has been noted in a wide variety of elasmobranchs e.g. smooth dogfish, (Mustelus canis) (Allee & Dickinson 1954), bonnethead sharks (Myrberg & Gruber 1974), lemon sharks (Gruber et al. 1988), scalloped hammerhead sharks, (Sphyrna lewini) (Klimley 1982, 1988), and white sharks (Fallows et al. 2013). Fallows, who studies the great white shark in South Africa, wrote in a personal communication (2022) that in more than thirty years he had never seen them fight, nor had he seen them bite each other while feeding together on a whale carcass. Nor yet have they reacted by biting when they have been lured to baits at cage diving boats and have mistakenly collided when swimming from one side of the boat to the other when they could not see each other.
Though mating wounds are inflicted on female blackfins (Porcher 2005) and females of other species while mating, males very rarely showed shallow teeth marks. No fighting, and no evidence of fighting, was seen.