Submitted:
06 September 2023
Posted:
07 September 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction:
Evolutionary argument:
Mechanistic argument:
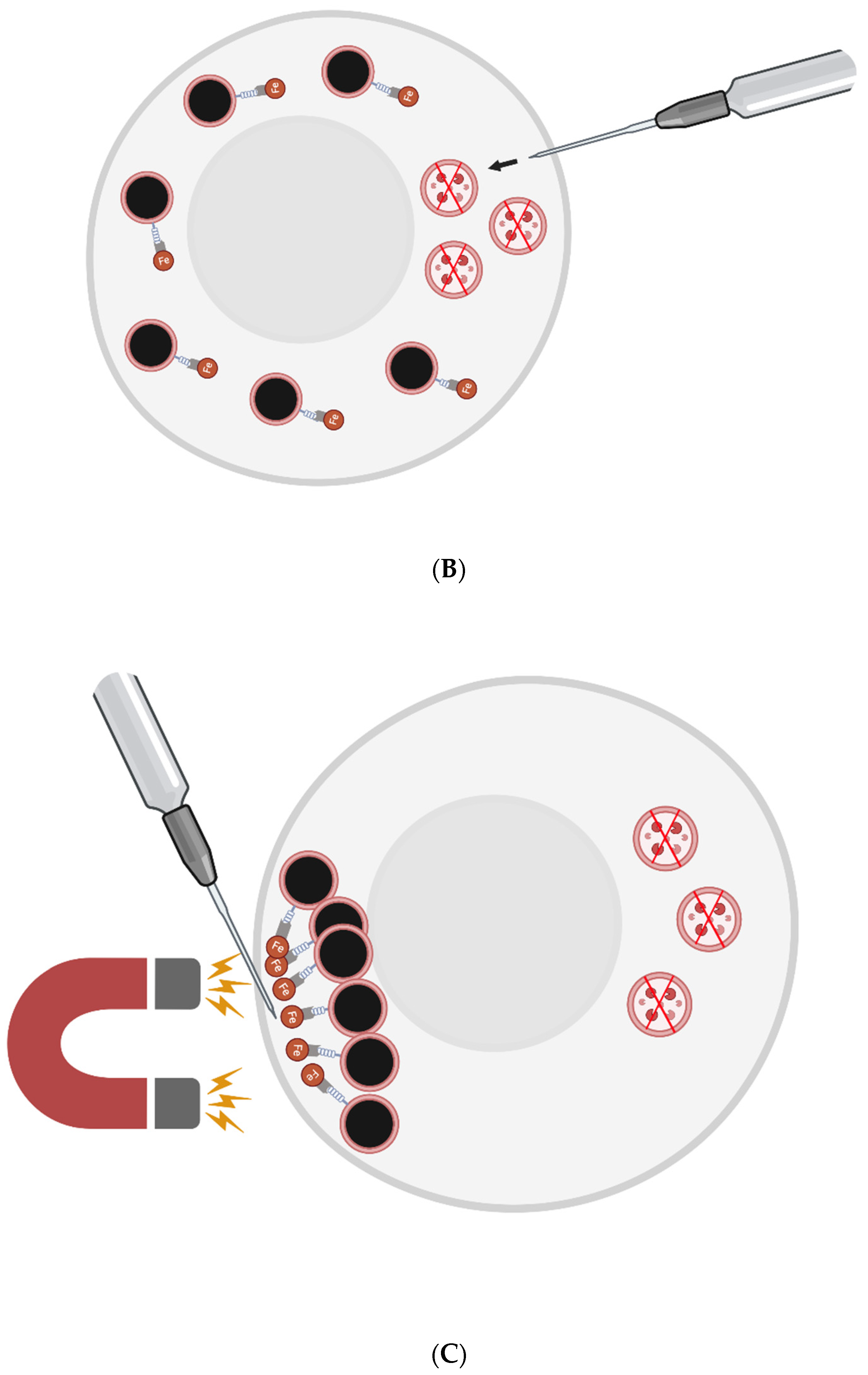
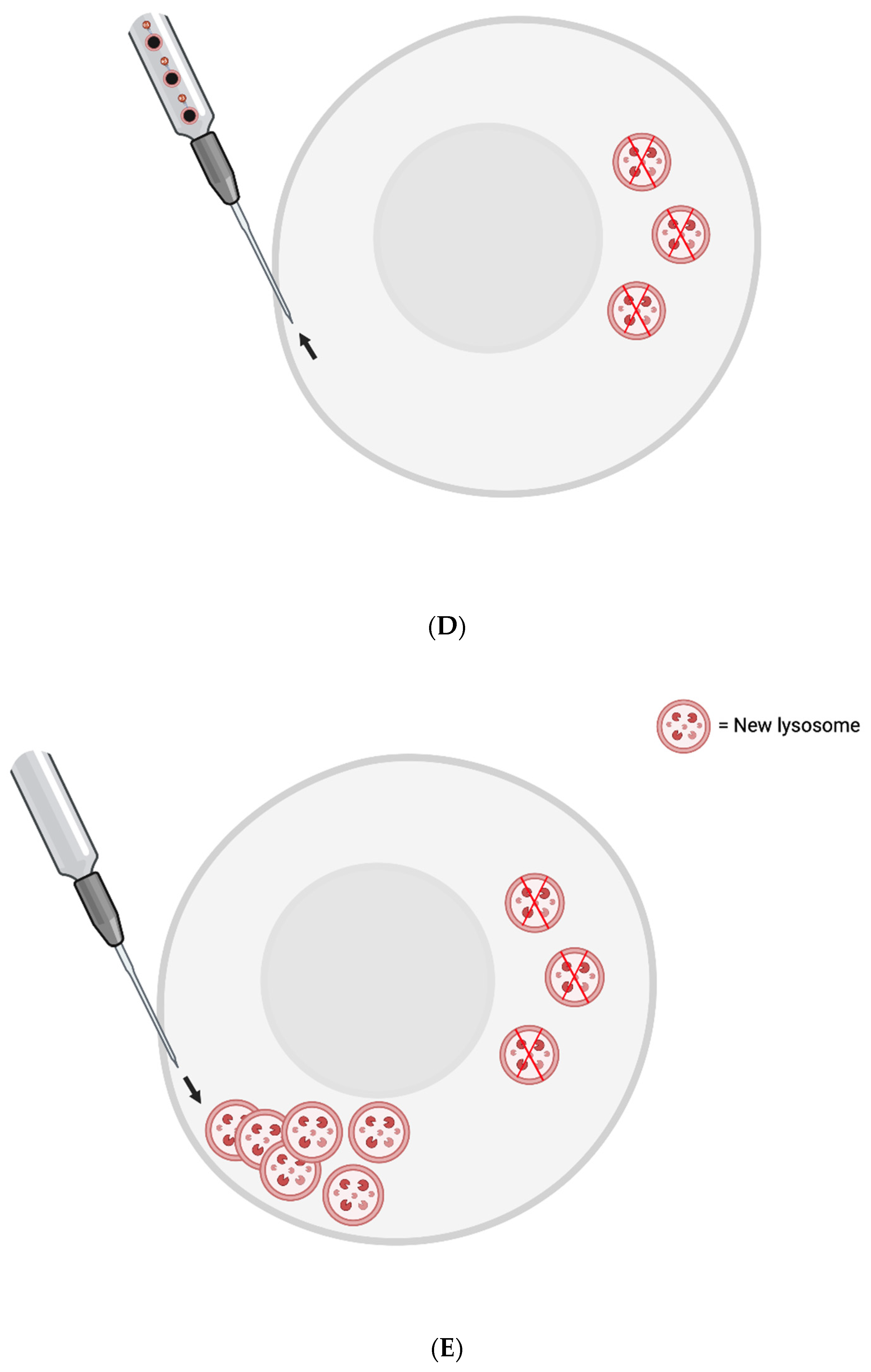
Testing the importance of lipofuscin accumulation in aging in vitro now:
Conclusion:
Ethics approval and consent to participate
Consent for publication
Availability of data and material
Competing interests
Authors' contributions
Funding
Acknowledgements
References
- Terman A and Brunk UT. Lipofuscin. The International Journal of Biochemistry & Cell Biology 2004;36(8):1400–1404. [CrossRef]
- Siebert S, Farrell JA, Cazet JF, et al. Stem Cell Differentiation Trajectories in Hydra Resolved at Single-Cell Resolution. Science 2019;365(6451):eaav9314. [CrossRef]
- Murad R, Macias-Muñoz A, Wong A, et al. Coordinated Gene Expression and Chromatin Regulation during Hydra Head Regeneration. Genome Biology and Evolution 2021;13(12):evab221. [CrossRef]
- Terman A, Brunk UT. Is aging the price for memory? Biogerontology (2005) 6:205–210. [CrossRef]
- Klapper W, Kühne K, Singh KK, Heidorn K, Parwaresch R, Krupp G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Letters (1998) 439:143–146. [CrossRef]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Structure & Development (2003) 32:39–60. [CrossRef]
- Peregrim I. Why we age — a new evolutionary view. Biologia (2017) 72:475–485. [CrossRef]
- Sheehy M, Shelton P, Wickins J, Belchier M, Gaten E. Ageing the European lobster Homarus gammarus by the lipofuscin in its eyestalk ganglia. Mar Ecol Prog Ser (1996) 143:99–111. [CrossRef]
- Kakimoto Y, Okada C, Kawabe N, Sasaki A, Tsukamoto H, Nagao R, Osawa M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci Rep (2019) 9:3304. [CrossRef]
- Karavanich C, Atema J. Individual recognition and memory in lobster dominance. Animal Behaviour (1998) 56:1553–1560. [CrossRef]
- Zhao S, Lin L, Kan G, Xu C, Tang Q, Yu C, Sun W, Cai L, Xu C, Cui S. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell Physiol Biochem (2014) 33:321–332. [CrossRef]
- Triplett JC, Tramutola A, Swomley A, Kirk J, Grimes K, Lewis K, Orr M, Rodriguez K, Cai J, Klein JB, et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: Implications promoting healthy longevity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease (2015) 1852:2213–2224. [CrossRef]
- Hadi F, Kulaberoglu Y, Lazarus KA, Bach K, Ugur R, Beattie P, Smith ESJ, Khaled WT. Transformation of naked mole-rat cells. Nature (2020) 583:E1–E7. [CrossRef]
- Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J (2011) 52:41–53. [CrossRef]
- Panno JP, Nair KK. Effects of increased lifespan on chromatin condensation in the adult male housefly. Mech Ageing Dev (1986) 35:31–38. [CrossRef]
- Clokey GV, Jacobson LA. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev (1986) 35:79–94. [CrossRef]
- Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp Gerontol (2002) 37:1015–1021. [CrossRef]
- Goyal VK. Lipofuscin pigment accumulation in the central nervous system of the mouse during aging. Exp Gerontol (1982) 17:89–94. [CrossRef]
- Gilissen EP, Staneva-Dobrovski L. Distinct Types of Lipofuscin Pigment in the Hippocampus and Cerebellum of Aged Cheirogaleid Primates. The Anatomical Record (2013) 296:1895–1906. [CrossRef]
- Moreno-García A, Kun A, Calero O, Medina M, Calero M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Frontiers in Neuroscience (2018) 12: https://www.frontiersin.org/article/10.3389/fnins.2018.00464 [Accessed March 31, 2022]. [CrossRef]
- Gray DA and Woulfe J. Lipofuscin and Aging: A Matter of Toxic Waste. Science of Aging Knowledge Environment 2005;2005(5):re1–re1. [CrossRef]
- Terman A, Brunk UT. Is Lipofuscin Eliminated from Cells? Investigative Ophthalmology & Visual Science (1999) 40:2463–2464.
- Wang L, Xiao C-Y, Li J-H, Tang G-C, Xiao S-S. Transport and Possible Outcome of Lipofuscin in Mouse Myocardium. Adv Gerontol 2022;12:247–63. [CrossRef]
- Burns JC, Cotleur B, Walther DM, Bajrami B, Rubino SJ, Wei R, Franchimont N, Cotman SL, Ransohoff RM, Mingueneau M. Differential accumulation of storage bodies with aging defines discrete subsets of microglia in the healthy brain. eLife (2020) 9:e57495. [CrossRef]
- Boellaard JW and Schlote W. Ultrastructural Heterogeneity of Neuronal Lipofuscin in the Normal Human Cerebral Cortex. Acta Neuropathol 1986;71(3–4):285–294. [CrossRef]
- Sohal RS, Wolfe LS. Chapter 11 Lipofuscin: characteristics and significance. In: Swaab DF, Fliers E, Mirmiran M, Van Gool WA, Van Haaren F, editors. Progress in Brain Research, vol. 70, Elsevier; 1986, p. 171–83. [CrossRef]
- Sheehy MRJ. Individual variation in, and the effect of rearing temperature and body size on, the concentration of fluorescent morphological lipofuscin in the brains of freshwater crayfish, Cherax cuspidatus (Crustacea: Parastacidae). Comparative Biochemistry and Physiology Part A: Physiology 1990;96:281–6. [CrossRef]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med (2002) 33:611–619. [CrossRef]
- Kurz T, Terman A, Gustafsson B, et al. Lysosomes and oxidative stress in aging and apoptosis. Biochimica et Biophysica Acta (BBA) - General Subjects 2008;1780(11):1291–1303. [CrossRef]
- Gabandé-Rodríguez E, Keane L, Capasso M. Microglial phagocytosis in aging and Alzheimer’s disease. Journal of Neuroscience Research 2020;98(2):284–298. [CrossRef]
- Pan C, Banerjee K, Lehmann GL, et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proceedings of the National Academy of Sciences 2021;118(47):e2100122118. [CrossRef]
- Terman A, Dalen H, Brunk UT. Ceroid/lipofuscin-loaded human fibroblasts show decreased survival time and diminished autophagocytosis during amino acid starvation☆. Experimental Gerontology 1999;34(8):943–957. [CrossRef]
- Terman A, Abrahamsson N, Brunk UT. Ceroid/lipofuscin-loaded human fibroblasts show increased susceptibility to oxidative stress. Exp Gerontol 1999;34(6):755–770. [CrossRef]
- Lv Z, Jiang H, Xu H, et al. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J Neural Transm 2011;118(3):361–369. [CrossRef]
- Maccarinelli F, Pagani A, Cozzi A, et al. A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiology of Disease 2015;81:119–133. [CrossRef]
- Bhoiwala D, Song Y, Cwanger A, et al. High iron diet causes elevation of retinal iron levels and RPE autofluorescence. Investigative Ophthalmology & Visual Science 2015;56(7):4203.
- Mangan D. Iron: an underrated factor in aging. Aging 2021;13(19):23407–23415. [CrossRef]
- hgami N, Yajima I, Iida M, et al. Manganese-mediated acceleration of age-related hearing loss in mice. Sci Rep 2016;6(1):36306. [CrossRef]
- Höhn A, Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol 2013;1(1):140–144. [CrossRef]
- von Zglinicki T, Nilsson E, Döcke WD, et al. Lipofuscin accumulation and ageing of fibroblasts. Gerontology 1995;41 Suppl 2:95–108. [CrossRef]
- Tsakiri EN, Iliaki KK, Höhn A, et al. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic Biol Med 2013;65:1155–1163. [CrossRef]
- de Grey AD. Appropriating Microbial Catabolism: A Proposal to Treat and Prevent Neurodegeneration. Neurobiology of aging 2006;27(4):589–595. [CrossRef]
- Sparrow JR, Parish CA, Hashimoto M, et al. A2E, a Lipofuscin Fluorophore, in Human Retinal Pigmented Epithelial Cells in Culture. Investigative Ophthalmology & Visual Science 1999;40(12):2988–2995.
- Anderson A, Campo A, Fulton E, et al. 7-Ketocholesterol in Disease and Aging. Redox Biology 2020;29:101380. [CrossRef]
- Seluanov A, Gladyshev VN, Vijg J, et al. Mechanisms of cancer resistance in long-lived mammals. Nat Rev Cancer 2018;18(7):433–441. [CrossRef]
- Samorajski T, Ordy JM, Rady-Reimer P. Lipofuscin pigment accumulation in the nervous system of aging mice. The Anatomical Record (1968) 160:555–573. [CrossRef]
- Brizzee KR, Johnson FA. Depth distribution of lipofuscin pigment in cerebral cortex of albino rat. Acta Neuropathol (1970) 16:205–219. [CrossRef]
- Yanai S, Endo S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Frontiers in Aging Neuroscience 2021;13. [CrossRef]
- Lutz CM, Osborne MA. Optimizing mouse models of neurodegenerative disorders: are therapeutics in sight? Future Neurology 2014;9(1):67–75. [CrossRef]
- Double KL, Dedov VN, Fedorow H, et al. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol Life Sci 2008;65(11):1669–1682. [CrossRef]
- Mann DM, Yates PO, Stamp JE. The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci (1978) 37:83–93. [CrossRef]
- Goyal VK. Lipofuscin pigment accumulation in human brain during aging. Experimental Gerontology 1982;17(6):481–487. [CrossRef]
- Benavides SH, Monserrat AJ, Fariña S, et al. Sequential histochemical studies of neuronal lipofuscin in human cerebral cortex from the first to the ninth decade of life. Archives of Gerontology and Geriatrics 2002;34(3):219–231. [CrossRef]
- Yin D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radical Biology and Medicine 1996;21(6):871–888. [CrossRef]
- Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investigative Ophthalmology & Visual Science (1978) 17:601–607.
- Dayan D, Abrahami I, Buchner A, Gorsky M, Chimovitz N. Lipid pigment (lipofuscin) in human perioral muscles with aging. Experimental Gerontology (1988) 23:97–102. [CrossRef]
- Xu H, Ren D. Lysosomal Physiology. Annu Rev Physiol (2015) 77:57–80. [CrossRef]
- López-Otín C, Blasco MA, Partridge L, et al. The Hallmarks of Aging. Cell 2013;153(6):1194–1217. [CrossRef]
- JACC: Cardiovascular Imaging, 1205. [CrossRef]
- Gumpenberger M, Wessner B, Graf A, et al. Remodeling the Skeletal Muscle Extracellular Matrix in Older Age—Effects of Acute Exercise Stimuli on Gene Expression. Int J Mol Sci 2020;21(19):7089. [CrossRef]
- Furber JD. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res 2006;9(2):274–278. [CrossRef]
- Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev 2020;34(23–24):1565–1576. [CrossRef]
- Guidi N, Marka G, Sakk V, et al. An Aged Bone Marrow Niche Restrains Rejuvenated Hematopoietic Stem Cells. STEM CELLS 2021;39(8):1101–1106. [CrossRef]
- Landspersky T, Saçma M, Rivière J, et al. Autophagy in Mesenchymal Progenitors Protects Mice against Bone Marrow Failure after Severe Intermittent Stress. Blood 2022;139(5):690–703. [CrossRef]
- Zhang X, Chen W, Gao Q, et al. Rapamycin Directly Activates Lysosomal Mucolipin TRP Channels Independent of MTOR. PLOS Biology 2019;17(5):e3000252. [CrossRef]
- Kabacik S, Lowe D, Fransen L, et al. The Relationship between Epigenetic Age and the Hallmarks of Aging in Human Cells. Nat Aging 2022;2(6):484–493. [CrossRef]
- Lu Y, Brommer B, Tian X, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020;588(7836):124–129. [CrossRef]
- Wang S, Xia P, Ye B, et al. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell 2013;13(5):617–625. [CrossRef]
- Kang, Y.-K., Min, B., Eom, J. & Park, J. S. Different phases of aging in mouse old skeletal muscle. Aging (Albany NY) 14, 143–160 (2022). [CrossRef]
- Vida C, de Toda IM, Cruces J, et al. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol 2017;12:423–437. [CrossRef]
- Settembre C, Di Malta C, Polito VA, et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011;332(6036):1429–1433. [CrossRef]
- Wang H, Wang R, Carrera I, et al. TFEB Overexpression in the P301S Model of Tauopathy Mitigates Increased PHF1 Levels and Lipofuscin Puncta and Rescues Memory Deficits. eNeuro 2016;3(2). [CrossRef]
- Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. Journal of Cell Biology (2002) 159:625–635. [CrossRef]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev Cell (2011) 21:421–430. [CrossRef]
- Julien S, Schraermeyer U. Lipofuscin can be eliminated from the retinal pigment epithelium of monkeys. Neurobiol Aging (2012) 33:2390–2397. [CrossRef]
- Gäbelein CG, Feng Q, Sarajlic E, Zambelli T, Guillaume-Gentil O, Kornmann B, Vorholt JA. Mitochondria transplantation between living cells. PLOS Biology (2022) 20:e3001576. [CrossRef]
- Beregi E, Regius O, Hüttl T, Göbl Z. Age-related changes in the skeletal muscle cells. Z Gerontol 1988;21:83–6.
- Hütter E, Skovbro M, Lener B, Prats C, Rabøl R, Dela F, et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 2007;6:245–56. [CrossRef]
- Triolo M, Hood DA. Manifestations of Age on Autophagy, Mitophagy and Lysosomes in Skeletal Muscle. Cells (2021) 10:1054. [CrossRef]
- Dong W, Cheng S, Huang F, Fan W, Chen Y, Shi H, He H. Mitochondrial dysfunction in long-term neuronal cultures mimics changes with aging. Med Sci Monit (2011) 17:BR91–BR96. [CrossRef]
- Moreno-Blas D, Gorostieta-Salas E, Pommer-Alba A, Muciño-Hernández G, Gerónimo-Olvera C, Maciel-Barón LA, Konigsberg M, Massieu L, Castro-Obregón S. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging (Albany NY) (2019) 11:6175–6198. [CrossRef]
- Li Z. “5.43 - In Vitro Micro-Tissue and -Organ Models for Toxicity Testing.,” In: Moo-Young M, editor. Comprehensive Biotechnology (Second Edition). Burlington: Academic Press (2011). p. 551–563. [CrossRef]
- Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. Journal of Neuroscience Methods (2001) 110:17–24. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



