Submitted:
04 April 2024
Posted:
08 April 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
Evolutionary Argument
Mechanistic Argument
Testing the Importance of Lipofuscin Accumulation in Aging In Vitro Now
Proof-of-Concept In Vivo
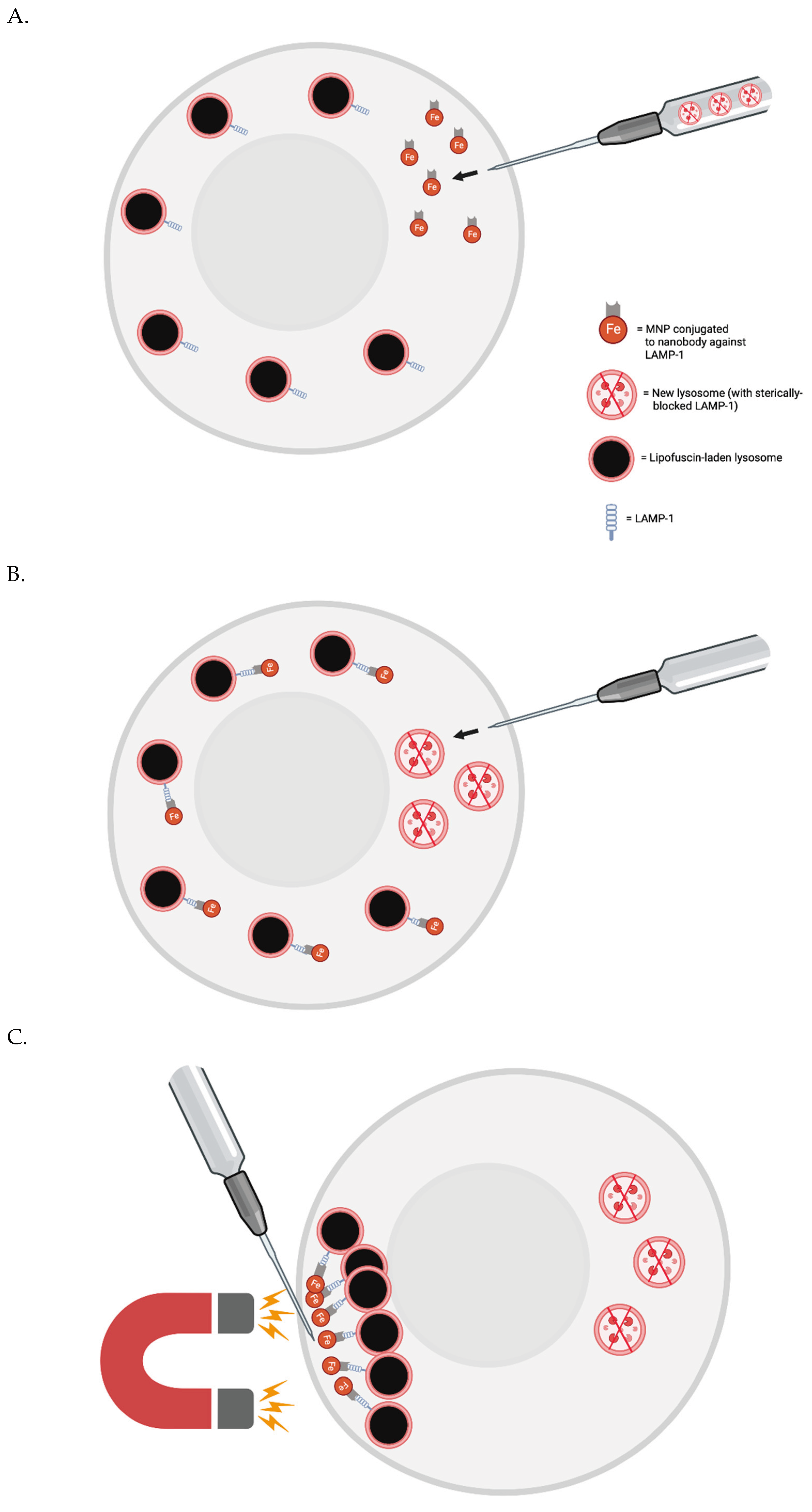
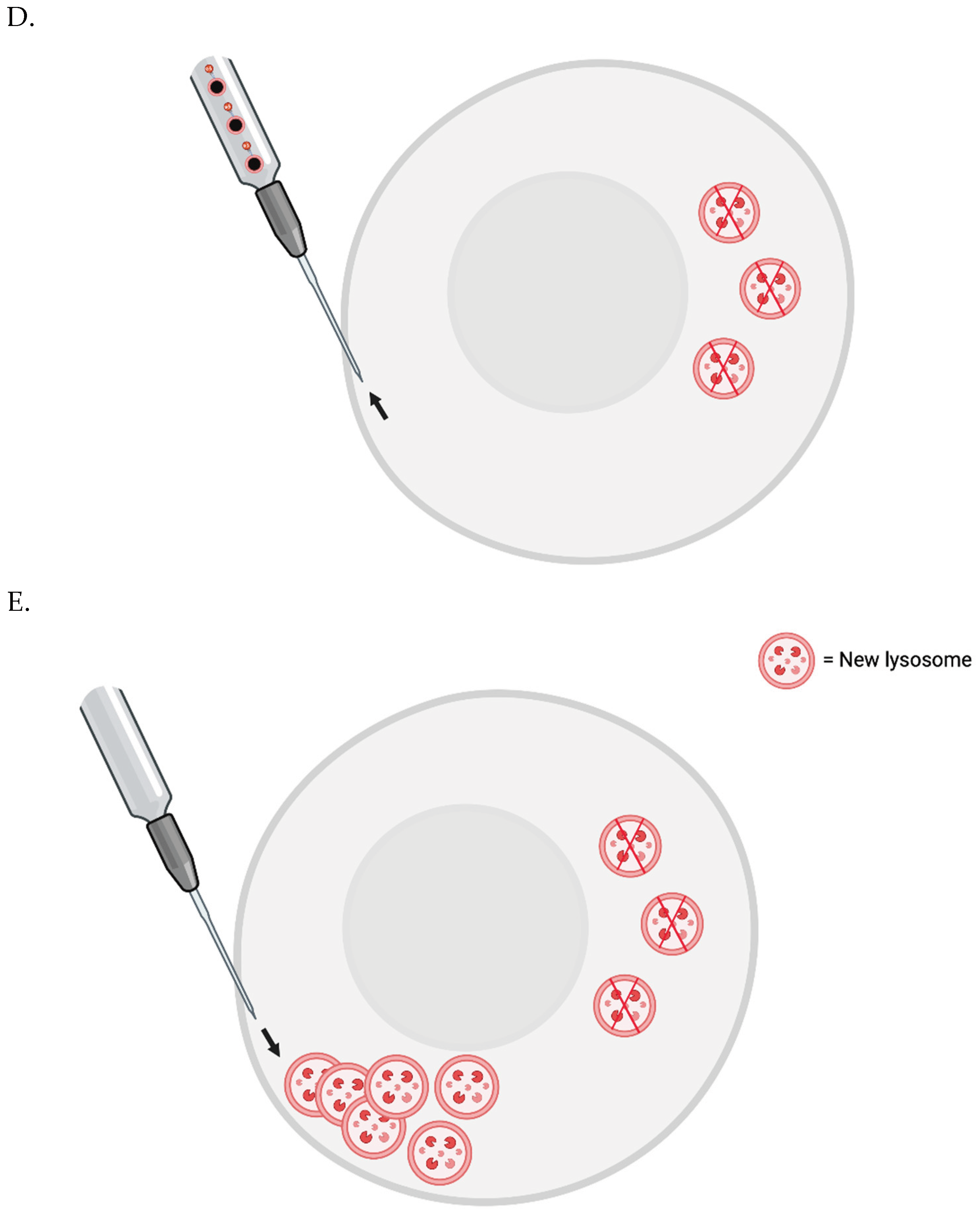
Periodic whole-body lipofuscin removal:
Periodic, Systemic Senescent Cell Destruction
Additional Longevity Therapies THAT Involve “Tweaking Metabolism”
*Sequencing Our Mitochondrial and Nuclear DNA as Soon as Possible
*Possibly Saving Cells with Pristine DNA as Soon as Possible
Eventually Fixing Nuclear and Mitochondrial Mutations
WILT
Conclusion
Funding
Authors’ Contributions
Ethics Approval and Consent to Participate
Consent for Publication
Availability of Data and Material
Acknowledgements
Competing Interests
References
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004, 36, 1400–1404. [Google Scholar] [CrossRef]
- Siebert, S.; Farrell, J.A.; Cazet, J.F.; Abeykoon, Y.; Primack, A.S.; Schnitzler, C.E.; Juliano, C.E. Stem Cell Differentiation Trajectories in Hydra Resolved at Single-Cell Resolution. Science 2019, 365, eaav9314. [Google Scholar] [CrossRef] [PubMed]
- Murad, R.; Macias-Muñoz, A.; Wong, A.; Ma, X.; Mortazavi, A. Coordinated Gene Expression and Chromatin Regulation during Hydra Head Regeneration. Genome Biol. Evol. 2021, 13, evab221. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Is aging the price for memory? Biogerontology 2005, 6, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W.; Kühne, K.; Singh, K.K.; Heidorn, K.; Parwaresch, R.; Krupp, G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 1998, 439, 143–146. [Google Scholar] [CrossRef]
- Beltz, B.S.; Sandeman, D.C. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Struct. Dev. 2003, 32, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Peregrim, I. Why we age—A new evolutionary view. Biologia 2017, 72, 475–485. [Google Scholar] [CrossRef]
- Sheehy, M.; Shelton, P.; Wickins, J.; Belchier, M.; Gaten, E. Ageing the European lobster Homarus gammarus by the lipofuscin in its eyestalk ganglia. Mar. Ecol. Prog. Ser. 1996, 143, 99–111. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef]
- Karavanich, C.; Atema, J. Individual recognition and memory in lobster dominance. Anim. Behav. 1998, 56, 1553–1560. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, L.; Kan, G.; Xu, C.; Tang, Q.; Yu, C.; Sun, W.; Cai, L.; Xu, C.; Cui, S. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell. Physiol. Biochem. 2014, 33, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Triplett, J.C.; Tramutola, A.; Swomley, A.; Kirk, J.; Grimes, K.; Lewis, K.; Orr, M.; Rodriguez, K.; Cai, J.; Klein, J.B.; et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: Implications promoting healthy longevity. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2015, 1852, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Kulaberoglu, Y.; Lazarus, K.A.; Bach, K.; Ugur, R.; Beattie, P.; Smith, E.S.J.; Khaled, W.T. Transformation of naked mole-rat cells. Nature 2020, 583, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Edrey, Y.H.; Hanes, M.; Pinto, M.; Mele, J.; Buffenstein, R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical. ILAR J. 2011, 52, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Panno, J.; Nair, K. Effects of increased lifespan on chromatin condensation in the adult male housefly. Mech. Ageing Dev. 1986, 35, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Clokey, G.V.; Jacobson, L.A. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech. Ageing Dev. 1986, 35, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Houthoofd, K.; Braeckman, B.P.; Lenaerts, I.; Brys, K.; De Vreese, A.; Van Eygen, S.; Vanfleteren, J.R. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp. Gerontol. 2002, 37, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Goyal, V.K. Lipofuscin pigment accumulation in the central nervous system of the mouse during aging. Exp. Gerontol. 1982, 17, 89–94. [Google Scholar] [CrossRef]
- Gilissen, E.P.; Staneva-Dobrovski, L. Distinct Types of Lipofuscin Pigment in the Hippocampus and Cerebellum of Aged Cheirogaleid Primates. Anat. Rec. 2013, 296, 1895–1906. [Google Scholar] [CrossRef]
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464. Available online: https://www.frontiersin.org/article/10.3389/fnins.2018.00464 (accessed on 31 March 2022). [CrossRef]
- Gray, D.A.; Woulfe, J. Lipofuscin and Aging: A Matter of Toxic Waste. Sci. Aging Knowl. Environ. 2005, 2005, re1. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. Is Lipofuscin Eliminated from Cells? Investigative Ophthalmology & Visual Science 1999, 40, 2463–2464. [Google Scholar]
- Wang, L.; Xiao, C.-Y.; Li, J.-H.; Tang, G.-C.; Xiao, S.-S. Transport and Possible Outcome of Lipofuscin in Mouse Myocardium. Adv. Gerontol. 2022, 12, 247–263. [Google Scholar] [CrossRef]
- Burns, J.C.; Cotleur, B.; Walther, D.M.; Bajrami, B.; Rubino, S.J.; Wei, R.; Franchimont, N.; Cotman, S.L.; Ransohoff, R.M.; Mingueneau, M. Differential accumulation of storage bodies with aging defines discrete subsets of microglia in the healthy brain. eLife 2020, 9, e57495. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, J.W.; Schlote, W. Ultrastructural Heterogeneity of Neuronal Lipofuscin in the Normal Human Cerebral Cortex. Acta Neuropathol. 1986, 71, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Wolfe, L.S. Chapter 11 Lipofuscin: characteristics and significance. In Progress in Brain Research; Swaab, D.F., Fliers, E., Mirmiran, M., Van Gool, W.A., Van Haaren, F., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 70, pp. 171–183. [Google Scholar] [CrossRef]
- Sheehy, M.R.J. Individual variation in, and the effect of rearing temperature and body size on, the concentration of fluorescent morphological lipofuscin in the brains of freshwater crayfish, Cherax cuspidatus (Crustacea: Parastacidae). Comp. Biochem. Physiol. Part A: Physiol. 1990, 96, 281–286. [Google Scholar] [CrossRef]
- Brunk, U.T.; Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002, 33, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2008, 1780, 1291–1303. [Google Scholar] [CrossRef]
- Gabandé-Rodríguez, E.; Keane, L.; Capasso, M. Microglial phagocytosis in aging and Alzheimer's disease. J. Neurosci. Res. 2020, 98, 284–298. [Google Scholar] [CrossRef]
- Pan, C.; Banerjee, K.; Lehmann, G.L.; Almeida, D.; Hajjar, K.A.; Benedicto, I.; Jiang, Z.; Radu, R.A.; Thompson, D.H.; Rodriguez-Boulan, E.; et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc. Natl. Acad. Sci. 2021, 118, e2100122118. [Google Scholar] [CrossRef]
- Terman, A.; Dalen, H.; Brunk, U.T. Ceroid/lipofuscin-loaded human fibroblasts show decreased survival time and diminished autophagocytosis during amino acid starvation☆. Exp. Gerontol. 1999, 34, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Abrahamsson, N.; Brunk, U.T. Ceroid/lipofuscin-loaded human fibroblasts show increased susceptibility to oxidative stress. Exp. Gerontol. 1999, 34, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Jiang, H.; Xu, H.; Song, N.; Xie, J. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J. Neural Transm. 2011, 118, 361–369. [Google Scholar] [CrossRef]
- Maccarinelli, F.; Pagani, A.; Cozzi, A.; Codazzi, F.; Di Giacomo, G.; Capoccia, S.; Rapino, S.; Finazzi, D.; Politi, L.S.; Cirulli, F.; et al. A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiol. Dis. 2015, 81, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Bhoiwala, D.; Song, Y.; Cwanger, A.; et al. High iron diet causes elevation of retinal iron levels and RPE autofluorescence. Investigative Ophthalmology & Visual Science 2015, 56, 4203. [Google Scholar]
- Mangan, D. Iron: an underrated factor in aging. Aging 2021, 13, 23407–23415. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, N.; Yajima, I.; Iida, M.; Li, X.; Oshino, R.; Kumasaka, M.Y.; Kato, M. Manganese-mediated acceleration of age-related hearing loss in mice. Sci. Rep. 2016, 6, 36306. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Grune, T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T.; Nilsson, E.; Döcke, W.D.; Brunk, U.T. LIPOFUSCIN Lipofuscin accumulation and ageing of fibroblasts. Gerontology 1995, 41 (Suppl. 2), 95–108. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Iliaki, K.K.; Höhn, A.; Grimm, S.; Papassideri, I.S.; Grune, T.; Trougakos, I.P. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free. Radic. Biol. Med. 2013, 65, 1155–1163. [Google Scholar] [CrossRef]
- Seluanov, A.; Gladyshev, V.N.; Vijg, J.; Gorbunova, V. Mechanisms of cancer resistance in long-lived mammals. Nat. Rev. Cancer 2018, 18, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Samorajski, T.; Ordy, J.M.; Rady-Reimer, P. Lipofuscin pigment accumulationin the nervous system of aging mice. Anat. Rec. 1968, 160, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Brizzee, K.R.; Johnson, F.A. Depth distribution of lipofuscin pigment in cerebral cortex of albino rat. Acta Neuropathol. 1970, 16, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Yanai, S.; Endo, S. Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype. Front. Aging Neurosci. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.M.; Osborne, M.A.; Anisimov, S.V.; Donnelly, C.J.; Grima, J.C.; Sattler, R.; Monani, U.R.; De Vivo, D.C.; Sturrock, A.; Leavitt, B.R.; et al. Optimizing mouse models of neurodegenerative disorders: are therapeutics in sight? Futur. Neurol. 2014, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Double, K.L.; Dedov, V.N.; Fedorow, H.; Kettle, E.; Halliday, G.M.; Garner, B.; Brunk, U.T. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell. Mol. Life Sci. 2008, 65, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; Yates, P.O.; Stamp, J.E. The relationship between lipofuscin pigment and ageing in the human nervous system. J. Neurol. Sci. 1978, 37, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Goyal, V.K. Lipofuscin pigment accumulation in human brain during aging. Exp. Gerontol. 1982, 17, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.H.; Monserrat, A.J.; Fariña, S.; et al. Sequential histochemical studies of neuronal lipofuscin in human cerebral cortex from the first to the ninth decade of life. Arch. Gerontol. Geriatr. 2002, 34, 219–231. [Google Scholar] [CrossRef]
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free. Radic. Biol. Med. 1996, 21, 871–888. [Google Scholar] [CrossRef]
- Wing, G.L.; Blanchard, G.C.; Weiter, J.J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Investigative Ophthalmology & Visual Science 1978, 17, 601–607. [Google Scholar]
- Dayan, D.; Abrahami, I.; Buchner, A.; Gorsky, M.; Chimovitz, N. Lipid pigment (lipofuscin) in human perioral muscles with aging. Exp. Gerontol. 1988, 23, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Min, B.; Eom, J.; Park, J.S. Different phases of aging in mouse old skeletal muscle. Aging 2022, 14, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Furber, J.D. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res. 2006, 9, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, R.; Carrera, I.; Xu, S.; Lakshmana, M.K. TFEB Overexpression in the P301S Model of Tauopathy Mitigates Increased PHF1 Levels and Lipofuscin Puncta and Rescues Memory Deficits. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K.; Andrews, N.W.; Simon, S.M. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002, 159, 625–635. [Google Scholar] [CrossRef]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Julien, S.; Schraermeyer, U. Lipofuscin can be eliminated from the retinal pigment epithelium of monkeys. Neurobiol. Aging 2012, 33, 2390–2397. [Google Scholar] [CrossRef]
- Gäbelein, C.G.; Feng, Q.; Sarajlic, E.; Zambelli, T.; Guillaume-Gentil, O.; Kornmann, B.; Vorholt, J.A. Mitochondria transplantation between living cells. PLOS Biol. 2022, 20, e3001576. [Google Scholar] [CrossRef]
- Beregi, E.; Regius, O.; Hüttl, T.; Göbl, Z. Age-related changes in the skeletal muscle cells. Z Gerontol 1988, 21, 83–6. [Google Scholar]
- Hütter, E.; Skovbro, M.; Lener, B.; Prats, C.; Rabøl, R.; Dela, F.; Jansen-Dürr, P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 2007, 6, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Hood, D.A. Manifestations of Age on Autophagy, Mitophagy and Lysosomes in Skeletal Muscle. Cells 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Cheng, S.; Huang, F.; Fan, W.; Chen, Y.; Shi, H.; He, H. Mitochondrial dysfunction in long-term neuronal cultures mimics changes with aging. Med Sci. Monit. 2011, 17, BR91–BR96. [Google Scholar] [CrossRef]
- Moreno-Blas, D.; Gorostieta-Salas, E.; Pommer-Alba, A.; Muciño-Hernández, G.; Gerónimo-Olvera, C.; Maciel-Barón, L.A.; Konigsberg, M.; Massieu, L.; Castro-Obregón, S. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging 2019, 11, 6175–6198. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. 5.43—In Vitro Micro-Tissue and -Organ Models for Toxicity Testing. In Comprehensive Biotechnology (Second Edition); Moo-Young, M., Ed.; Academic Press: Burlington, 2011; pp. 551–563. [Google Scholar] [CrossRef]
- Potter, S.M.; DeMarse, T.B. A new approach to neural cell culture for long-term studies. J. Neurosci. Methods 2001, 110, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kraner-Scheiber, S.; Petersen, B.; Rieblinger, B.; Buermann, A.; Flisikowska, T.; Flisikowski, K.; Christan, S.; Edlinger, M.; Baars, W.; et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci. Rep. 2016, 6, 29081. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNARE s and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef]
- Scheller, L.; Strittmatter, T.; Fuchs, D.; Bojar, D.; Fussenegger, M. Generalized extracellular molecule sensor platform for programming cellular behavior. Nat. Chem. Biol. 2018, 14, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Kim, B.; Byun, J.-W.; Baik, S.H.; Huh, Y.H.; Kim, J.-H.; Mook-Jung, I.; Song, W.K.; Shin, J.-H.; Seo, H.; et al. LRRK2 G2019S mutation attenuates microglial motility by inhibiting focal adhesion kinase. Nat. Commun. 2015, 6, 8255. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, G.B.; Cunningham, P.S.; Poolman, T.M.; Iqbal, M.; Maidstone, R.; Baxter, M.; Bagnall, J.; Begley, N.; Saer, B.; Hussell, T.; et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA 2020, 117, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 as a target for modulating immune system responses. Neurobiol. Dis. 2022, 169, 105724. [Google Scholar] [CrossRef] [PubMed]
- Insall, R.H.; Paschke, P.; Tweedy, L. Steering yourself by the bootstraps: how cells create their own gradients for chemotaxis. Trends Cell Biol. 2022, 32, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Tigges, M.; Marquez-Lago, T.T.; Stelling, J.; Fussenegger, M. A tunable synthetic mammalian oscillator. Nature 2009, 457, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.S.; Lu, G.; Dong, H.; Cho, Y.-L.; Natalia, A.; Wang, L.; Chan, C.; Kappei, D.; Taneja, R.; Ling, S.-C.; et al. A degradative to secretory autophagy switch mediates mitochondria clearance in the absence of the mATG8-conjugation machinery. Nat. Commun. 2022, 13, 3720. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.A.; Williamson, A.P.; Steinbach, A.M.; Roberts, E.W.; Kern, N.; Headley, M.B.; Vale, R.D. Chimeric antigen receptors that trigger phagocytosis. eLife 2018, 7, e36688. [Google Scholar] [CrossRef]
- Birgisdottir, Å.B.; Lamark, T.; Johansen, T. The LIR motif – crucial for selective autophagy. J. Cell Sci. 2013, 126 Pt 15, 3237–3247. [Google Scholar] [CrossRef]
- Uematsu, M.; Nishimura, T.; Sakamaki, Y.; Yamamoto, H.; Mizushima, N. Accumulation of undegraded autophagosomes by expression of dominant-negative STX17 (syntaxin 17) mutants. Autophagy 2017, 13, 1452–1464. [Google Scholar] [CrossRef]
- Friedland, A.E.; Lu, T.K.; Wang, X.; Shi, D.; Church, G.; Collins, J.J. Synthetic Gene Networks that Count. Science 2009, 324, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- VanHook, A.M. Macrophages don’t take more than they can eat. Sci. Signal. 2017, 10, eaao1183. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Rhau, B.; Hermann, A.; McNally, K.A.; Zhou, C.; Gong, D.; Weiner, O.D.; Conklin, B.R.; Onuffer, J.; Lim, W.A. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc. Natl. Acad. Sci. USA 2014, 111, 5896–5901. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Miyakawa, N.; Takuwa, H.; Hori, Y.; Oyama, K.; Ji, B.; Takahashi, M.; Huang, X.-P.; Slocum, S.T.; DiBerto, J.F.; et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020, 23, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Ronda, C.; Chen, S.P.; Cabral, V.; Yaung, S.J.; Wang, H.H. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods 2019, 16, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maxwell, K.G.; Wang, K.; Bowers, D.T.; Flanders, J.A.; Liu, W.; Wang, L.-H.; Liu, Q.; Liu, C.; Naji, A.; et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci. Transl. Med. 2021, 13, eabb4601. [Google Scholar] [CrossRef] [PubMed]
- Spampanato, C.; Feeney, E.; Li, L.; Cardone, M.; Lim, J.-A.; Annunziata, F.; Zare, H.; Polishchuk, R.; Puertollano, R.; Parenti, G.; et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 2013, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.Y.W.; Hayase, N.; Yuen, P.S.T.; Lee, J.; Fernandez, K.; Hu, X.; et al. Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lund, H.; Pieber, M.; Parsa, R.; Han, J.; Grommisch, D.; Ewing, E.; Kular, L.; Needhamsen, M.; Espinosa, A.; Nilsson, E.; et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 2018, 9, 4845. [Google Scholar] [CrossRef]
- Gustafsson, K.; Rhee, C.; Frodermann, V.; Scadden, E.W.; Li, D.; Iwamoto, Y.; Palchaudhuri, R.; Hyzy, S.L.; Boitano, A.E.; Nahrendorf, M.; et al. Clearing and replacing tissue-resident myeloid cells with an anti-CD45 antibody–drug conjugate. Blood Adv. 2023, 7, 6964–6973. [Google Scholar] [CrossRef]
- Butowski, N.; Colman, H.; De Groot, J.F.; Omuro, A.M.; Nayak, L.; Wen, P.Y.; Cloughesy, T.F.; Marimuthu, A.; Haidar, S.; Perry, A.; et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-Oncology 2016, 18, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Green, K.N.; Crapser, J.D.; Hohsfield, L.A. To Kill Microglia: A Case for CSF1R Inhibitors. Trends Immunol. 2020, 41, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Gil-Martin, M.; Bauer, T.M.; Naing, A.; Lim, D.W.-T.; Sarantopoulos, J.; Geva, R.; Ando, Y.; Fan, L.; Choudhury, S.; et al. Abstract CT171: Phase I study of BLZ945 alone and with spartalizumab (PDR001) in patients (pts) with advanced solid tumors. Cancer Res 2020, 80, CT171. [Google Scholar] [CrossRef]
- Krance, R.A.; Kuehnle, I.; Rill, D.R.; Mei, Z.; Pinetta, C.; Evans, W.; Brown, M.P.; Pulé, M.; Heslop, H.E.; Brenner, M.K. Hematopoietic and immunomodulatory effects of lytic CD45 monoclonal antibodies in patients with hematologic malignancy. Biol. Blood Marrow Transplant. 2003, 9, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Rafiei Hashtchin, A.; Manstein, F.; Carvalho Oliveira, M.; Kempf, H.; Zweigerdt, R.; et al. Continuous human iPSC-macrophage production by suspension culture in stirred tank bioreactors. Nat. Protoc. 2022, 17, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Fattorelli, N.; Martinez-Muriana, A.; Wolfs, L.; Geric, I.; De Strooper, B.; Mancuso, R. Stem-cell-derived human microglia transplanted into mouse brain to study human disease. Nat. Protoc. 2021, 16, 1013–1033. [Google Scholar] [CrossRef]
- Cao, X.; Yakala, G.K.; van den Hil, F.E.; Cochrane, A.; Mummery, C.L.; Orlova, V.V. Differentiation and Functional Comparison of Monocytes and Macrophages from hiPSCs with Peripheral Blood Derivatives. Stem Cell Rep. 2019, 12, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 2022, 7, eabf7777. [Google Scholar] [CrossRef] [PubMed]
- Palchaudhuri, R.; Saez, B.; Hoggatt, J.; Schajnovitz, A.; Sykes, D.B.; Tate, T.A.; Czechowicz, A.; Kfoury, Y.; Ruchika, F.; Rossi, D.J.; et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat. Biotechnol. 2016, 34, 738–745. [Google Scholar] [CrossRef]
- Breda, L.; Papp, T.E.; Triebwasser, M.P.; Yadegari, A.; Fedorky, M.T.; Tanaka, N.; Abdulmalik, O.; Pavani, G.; Wang, Y.; Grupp, S.A.; et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 2023, 381, 436–443. [Google Scholar] [CrossRef]
- Wellhausen, N.; O’Connell, R.P.; Lesch, S.; Engel, N.W.; Rennels, A.K.; Gonzales, D.; Herbst, F.; Young, R.M.; Garcia, K.C.; Weiner, D.; et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 2023, 15, eadi1145. [Google Scholar] [CrossRef] [PubMed]
- Horns, F.; Martinez, J.A.; Fan, C.; Haque, M.; Linton, J.M.; Tobin, V.; Santat, L.; Maggiolo, A.O.; Bjorkman, P.J.; Lois, C.; et al. Engineering RNA export for measurement and manipulation of living cells. Cell 2023, 186, 3642–3658.e32. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, C.T.; Homma, S.; Suchy, F.; Wang, S.; Bhadhury, J.; Amaya, A.K.; et al. Secreted Particle Information Transfer (SPIT)—A Cellular Platform for In Vivo Genetic Engineering. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mc Cafferty, S.; De Temmerman, J.; Kitada, T.; Becraft, J.R.; Weiss, R.; Irvine, D.J.; Devreese, M.; De Baere, S.; Combes, F.; Sanders, N.N. In Vivo Validation of a Reversible Small Molecule-Based Switch for Synthetic Self-Amplifying mRNA Regulation. Mol. Ther. 2021, 29, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, M.; Gawletta, S.; Hempel, T.; Brill, S.; Nett, E.; Sahin, U.; Beissert, T. A trans-amplifying RNA simplified to essential elements is highly replicative and robustly immunogenic in mice. Mol. Ther. 2023, 31, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Moss, B. Infectious poxvirus vectors have capacity for at least 25,000 base pairs of foreign DNA. Gene 1983, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.E.; Oggier, A.; Füglistaler, A.; Camviel, N.; Hijazi, M.; Villarreal, A.R.; Arber, C.; Barth, P. Computational design of dynamic receptor—peptide signaling complexes applied to chemotaxis. Nat. Commun. 2023, 14, 2875. [Google Scholar] [CrossRef]
- Saberi, A.; Gulyaeva, A.A.; Brubacher, J.L.; Newmark, P.A.; Gorbalenya, A.E. A planarian nidovirus expands the limits of RNA genome size. PLOS Pathog. 2018, 14, e1007314. [Google Scholar] [CrossRef]
- Zhu, J.; Batra, H.; Ananthaswamy, N.; Mahalingam, M.; Tao, P.; Wu, X.; Guo, W.; Fokine, A.; Rao, V.B. Design of bacteriophage T4-based artificial viral vectors for human genome remodeling. Nat. Commun. 2023, 14, 2928. [Google Scholar] [CrossRef]
- Hohsfield, L.A.; Najafi, A.R.; Ghorbanian, Y.; Soni, N.; Hingco, E.E.; Kim, S.J.; Jue, A.D.; Swarup, V.; Inlay, M.A.; Green, K.N. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J. Neuroinflammation 2020, 17, 279. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.M.; Bhaskar, U.; Linhardt, R.J.; Dordick, J.S. Effect of eliminase gene (elmA) deletion on heparosan production and shedding in Escherichia coli K5. J. Biotechnol. 2013, 165, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-W.; Liu, Y.; Lee, Y.-Q.; Yang, P.-J.; Ho, C.-T.; Hong, J.-C.; Hsiao, J.-C.; Liao, D.-C.; Liang, A.-J.; Hung, T.-C.; et al. Construction of intracellular asymmetry and asymmetric division in Escherichia coli. Nat. Commun. 2021, 12, 888. [Google Scholar] [CrossRef]
- Szczesna, M.; Huang, Y.; Lacoursiere, R.E.; Bonini, F.; Pol, V.; Koc, F.; et al. Dedicated bacterial esterases reverse lipopolysaccharide ubiquitylation to block immune sensing. Res Sq 2023, rs.3.rs-2986327. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Bouton, C.R.; Hu, K.; Samnuan, K.; Shattock, R.J. Innate Inhibiting Proteins Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2021, 29, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Ferdosi, S.R.; Ewaisha, R.; Moghadam, F.; Krishna, S.; Park, J.G.; Ebrahimkhani, M.R.; Kiani, S.; Anderson, K.S. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun. 2019, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Muhuri, M.; Li, S.; Qin, W.; Xu, G.; Luo, L.; Li, J.; Letizia, A.J.; Wang, S.K.; Chan, Y.K.; et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight 4, e99052. [CrossRef] [PubMed]
- Yoo, J.-S.; Sasaki, M.; Cho, S.X.; Kasuga, Y.; Zhu, B.; Ouda, R.; Orba, Y.; de Figueiredo, P.; Sawa, H.; Kobayashi, K.S. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat. Commun. 2021, 12, 6602. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Martinez-Vicente, M.; Macian, F.; Verkhusha, V.V.; Cuervo, A.M. A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat. Commun. 2011, 2, 386. [Google Scholar] [CrossRef]
- Lonzarić, J.; Lebar, T.; Majerle, A.; Manček-Keber, M.; Jerala, R. Locked and proteolysis-based transcription activator-like effector (TALE) regulation. Nucleic Acids Res. 2016, 44, 1471–1481. [Google Scholar] [CrossRef]
- Slabaugh, E.; Brandizzi, F. Membrane-tethered transcription factors provide a connection between stress response and developmental pathways. Plant Signal. Behav. 2011, 6, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Rubens, J.R.; Selvaggio, G.; Lu, T.K. Synthetic mixed-signal computation in living cells. Nat. Commun. 2016, 7, 11658. [Google Scholar] [CrossRef] [PubMed]
- Streeter, M.D.; Rowan, S.; Ray, J.; McDonald, D.M.; Volkin, J.; Clark, J.; Taylor, A.; Spiegel, D.A. Generation and Characterization of Anti-Glucosepane Antibodies Enabling Direct Detection of Glucosepane in Retinal Tissue. ACS Chem. Biol. 2020, 15, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Send in the senolytics. Nat. Biotechnol. 2020, 38, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, D.; Sun, B.; Sun, B.; Li, S.; Li, S.; Wei, W.; Wei, W.; Liu, X.; Liu, X.; et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 2023, 15, eadd1951. [Google Scholar] [CrossRef]
- VitaDAO - ApoptoSENS - Senolytic CAR-NK Cells. Available online: https://www.vitadao.com/projects/apoptosens-senolytic-car-nk-cells (accessed on 3 April 2024).
- Kaseniit, K.E.; Katz, N.; Kolber, N.S.; Call, C.C.; Wengier, D.L.; Cody, W.B.; Sattely, E.S.; Gao, X.J. Modular, programmable RNA sensing using ADAR editing in living cells. Nat. Biotechnol. 2023, 41, 482–487. [Google Scholar] [CrossRef]
- Gayet, R.V.; Ilia, K.; Razavi, S.; Tippens, N.D.; Lalwani, M.A.; Zhang, K.; Chen, J.X.; Chen, J.C.; Vargas-Asencio, J.; Collins, J.J. Autocatalytic base editing for RNA-responsive translational control. Nat. Commun. 2023, 14, 1339. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef]
- Jaijyan, D.K.; Selariu, A.; Cruz-Cosme, R.; Tong, M.; Yang, S.; Stefa, A.; Kekich, D.; Sadoshima, J.; Herbig, U.; Tang, Q.; et al. New intranasal and injectable gene therapy for healthy life extension. Proc. Natl. Acad. Sci. USA 2022, 119, e2121499119. [Google Scholar] [CrossRef] [PubMed]
- Sewell, P.E. Systemic Human Htert Aav Gene Transfer Therapy And The Effect On Telomere Length And Biological Age, A Case Report. J. Regen. Biol. Med. 2022. [Google Scholar] [CrossRef]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Mattsson, B.; Weikop, P.; Lundblad, M.; Jakobsson, J.; Björklund, A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl. Acad. Sci. USA 2013, 110, E1817–E1826. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, N.; Nowell, C.S.; Blackburn, C.C. Regeneration of the aged thymus by a single transcription factor. Development 2014, 141, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- García-Cao, I.; García-Cao, M.; Martín-Caballero, J.; Criado, L.M.; Klatt, P.; Flores, J.M.; Weill, J.-C.; Blasco, M.A.; Serrano, M. 'Super p53' mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002, 21, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Matheu, A.; Maraver, A.; Klatt, P.; Flores, I.; Garcia-Cao, I.; Borras, C.; Flores, J.M.; Viña, J.; Blasco, M.A.; Serrano, M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007, 448, 375–379. [Google Scholar] [CrossRef]
- Zhao, Y.; Burikhanov, R.; Qiu, S.; Lele, S.M.; Jennings, C.D.; Bondada, S.; Spear, B.; Rangnekar, V.M. Cancer resistance in transgenic mice expressing the SAC module of Par-4. Cancer Res 2007, 67, 9276–9285. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Song, M.S.; Hobbs, R.M.; Laurent, G.; Giorgi, C.; de Boer, V.C.; Anastasiou, D.; Ito, K.; Sasaki, A.T.; Rameh, L.; et al. Systemic Elevation of PTEN Induces a Tumor-Suppressive Metabolic State. Cell 2012, 149, 49–62. [Google Scholar] [CrossRef]
- Bujarrabal-Dueso, A.; Sendtner, G.; Meyer, D.H.; Chatzinikolaou, G.; Stratigi, K.; Garinis, G.A.; Schumacher, B. The DREAM complex functions as conserved master regulator of somatic DNA-repair capacities. Nat. Struct. Mol. Biol. 2023, 30, 475–488. [Google Scholar] [CrossRef]
- Barroca, V.; Fouchet, P. Germline Stem Cells: The First Guards of Heredity. Cell Stem Cell 2008, 2, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wang, B.; Zhu, Y.; Wu, J.; Qiu, L.; Ling, S.; Zhou, Z.; Dai, Y.; Zhong, Z.; Zheng, Y. Female germline stem cells: aging and anti-aging. J. Ovarian Res. 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Winship, A.; Zerafa, N.; Wakefield, M.; Hutt, K. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proc. Natl. Acad. Sci. 2020, 117, 11513–11522. [Google Scholar] [CrossRef]
- St. John, J.C. Mitochondria and Female Germline Stem Cells—A Mitochondrial DNA Perspective. Cells 2019, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Lechuga-Vieco, A.V.; Latorre-Pellicer, A.; Calvo, E.; Torroja, C.; Pellico, J.; Acín-Pérez, R.; García-Gil, M.L.; Santos, A.; Bagwan, N.; Bonzon-Kulichenko, E.; et al. Heteroplasmy of Wild-Type Mitochondrial DNA Variants in Mice Causes Metabolic Heart Disease With Pulmonary Hypertension and Frailty. Circ. 2022, 145, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Pellicer, A.; Moreno-Loshuertos, R.; Lechuga-Vieco, A.V.; Sánchez-Cabo, F.; Torroja, C.; Acín-Pérez, R.; Calvo, E.; Aix, E.; González-Guerra, A.; Logan, A.; et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 2016, 535, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Zealley, B.; de Grey, A.D.N.J. Strategies for Engineered Negligible Senescence. Gerontology 2013, 59, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.G.; Koob, M.D. Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res. 2005, 33, e139. [Google Scholar] [CrossRef]
- Zekonyte, U.; Bacman, S.R.; Smith, J.; Shoop, W.; Pereira, C.V.; Tomberlin, G.; Stewart, J.; Jantz, D.; Moraes, C.T. Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo. Nat. Commun. 2021, 12, 3210. [Google Scholar] [CrossRef]
- Sato, T.K.; Kawano, S.; Endo, T. Role of the membrane potential in mitochondrial protein unfolding and import. Sci. Rep. 2019, 9, 7637. [Google Scholar] [CrossRef]
- Abascal, F.; Harvey, L.M.R.; Mitchell, E.; Lawson, A.R.J.; Lensing, S.V.; Ellis, P.; Russell, A.J.C.; Alcantara, R.E.; Baez-Ortega, A.; Wang, Y.; et al. Somatic mutation landscapes at single-molecule resolution. Nature 2021, 593, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.A.B.; Santostasi, G. Whole-Body Induced Cell Turnover: A Proposed Intervention for Age-Related Damage and Associated Pathology. Rejuvenation Res. 2016, 19, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Scheller, L.; Strittmatter, T.; Fuchs, D.; Bojar, D.; Fussenegger, M. Generalized extracellular molecule sensor platform for programming cellular behavior. Nat. Chem. Biol. 2018, 14, 723–729. [Google Scholar] [CrossRef]
- Renteln, M.A. Promoting Oncolytic Vector Replication with Switches that Detect Ubiquitous Mutations. Curr. Cancer Ther. Rev. 2024, 20, 40–52. [Google Scholar] [CrossRef]
- Renteln, M. A New Paradigm in Cancer Treatment: Identifying and Targeting Clonal Mutations 2024. [CrossRef]
- de Grey, A.D. Whole-body interdiction of lengthening of telomeres: a proposal for cancer prevention. Front. Biosci. 2005, 10, 2420–9. [Google Scholar] [CrossRef] [PubMed]
- Lansing, F.; Paszkowski-Rogacz, M.; Schmitt, L.T.; Schneider, P.M.; Romanos, T.R.; Sonntag, J.; Buchholz, F. A heterodimer of evolved designer-recombinases precisely excises a human genomic DNA locus. Nucleic Acids Res. 2020, 48, 472–485. [Google Scholar] [CrossRef]
- Sommer, A.; Royle, N.J. ALT: A Multi-Faceted Phenomenon. Genes 2020, 11, 133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



