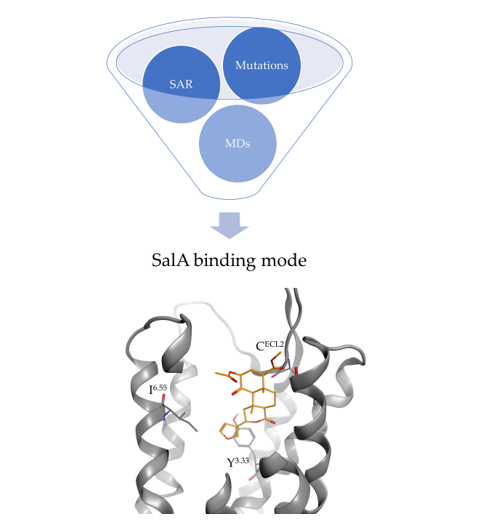
The natural product Salvinorin A (SalA) was the first nitrogen-lacking agonist discovered for the opioid receptors and exhibits high selectivity for the kappa opioid receptor (KOR) turning SalA into a promising analgesic to overcome the current opioid crisis. Since SalA’s suffers from poor pharmacokinetic properties, particularly the absence of gastrointestinal bioavailability, fast metabolic inactivation and subsequent short duration of action, the rational design of new tailored analogs with improved clinical usability is highly desired. Despite being known for decades, the binding mode of SalA within the KOR remains elusive as several conflicting binding modes of SalA were proposed hindering the rational design of new analgesics. In this study, we rationally determined the binding mode of SalA to the active state KOR by in silico experiments (docking, molecular dynamics simulations, dynophores) in the context of all available mutagenesis studies and structure-activity relationship (SAR) data. To the best of our knowledge this is the first comprehensive evaluation of SalA’s binding mode since the determination of the active state KOR crystal structure. SalA binds above the morphinan binding site with its furan pointing towards the intracellular core while the C2-acetoxy group is oriented towards the extracellular loop 2 (ECL2). SalA is solely stabilized within the binding pocket by hydrogen bonds (C210ECL2, Y3127.35, Y3137.36) and hydrophobic contacts (V1182.63, I1393.33, I2946.55, I3167.39). With the disruption of this interaction pattern or the establishment of additional interactions within the binding site we were able to rationalize the experimental data for selected analogs. We surmise the C2-substituent interactions as important for SalA and its analogs to be experimentally active, albeit moderate frequency within MD simulations of SalA. We further identified the non-conserved residues 2.63, 7.35, and 7.36 responsible for the KOR subtype selectivity of SalA. We are confident that the elucidation of the SalA binding mode will promote the understanding of KOR activation and the facilitate the development of novel analgesics that are urgently needed.