Preprint
Article
Evaluation of Serological Tests to Determine Postvaccinal Immunity to SARS-Cov-2 by Mrna Vaccines
Altmetrics
Downloads
174
Views
90
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
25 October 2022
Posted:
04 November 2022
You are already at the latest version
Alerts
Abstract
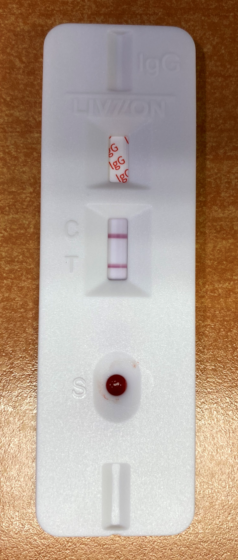
Background: The duration of the vaccine's protective efficacy against SARS-CoV-2 is unknown. Evaluation of the clinical performance of available tests is required. Objectives: To evaluate the clinical performance of three immunoassays for the detection of IgG antibodies generated by mRNA vaccines against SARS-CoV-2. Methods: Two automated immunoassays (Euroimmun Anti-SARS-CoV-2 ELISA IgG and Abbott SARS-CoV-2 CLIA IgG) and one lateral flow immunoassay (LFIA Test Livzon IgG) were tested. 300 samples distributed in 3 groups were analyzed: 100 subjects over 18 years old and under 45 years old, 100 subjects between 45-65 years old and another 100 over 65 years old. collected before vaccination, at 21 days, 1, 2, 3 and 6 months post-vaccination. Sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, and agreement (I. Kappa) were calculated for each serological test. Results: Maximum sensitivity for IgG was 98.7%, 98.1%, and 97.8% for the ELISA Euroimmun, CLIA Abbott, and Livzon LFIA assays and maximum specificity for IgG was 99.4%, 99.9%. % and 98.4% ELISA, CLIA and LFIA respectively at 3 months after vaccination with a decrease in antibody levels from the sixth month. The best agreement was observed between ELISA and CLIA 100%; (k = 1.00). The agreement between ELIA, CLIA and LIFIA was 99% (k = 0964) at the second and third month after vaccination. Seroconversion was faster and longer lasting in the younger age groups. Conclusion: Our study showed an equivalent and homogeneous clinical performance for IgG of three immunoassays after vaccination and that the LIFIA assay is the most cost-effective, reliable and accurate for routine use in population studies of seroconversion and seroprevalence.
Keywords:
Subject: Medicine and Pharmacology - Pathology and Pathobiology
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






