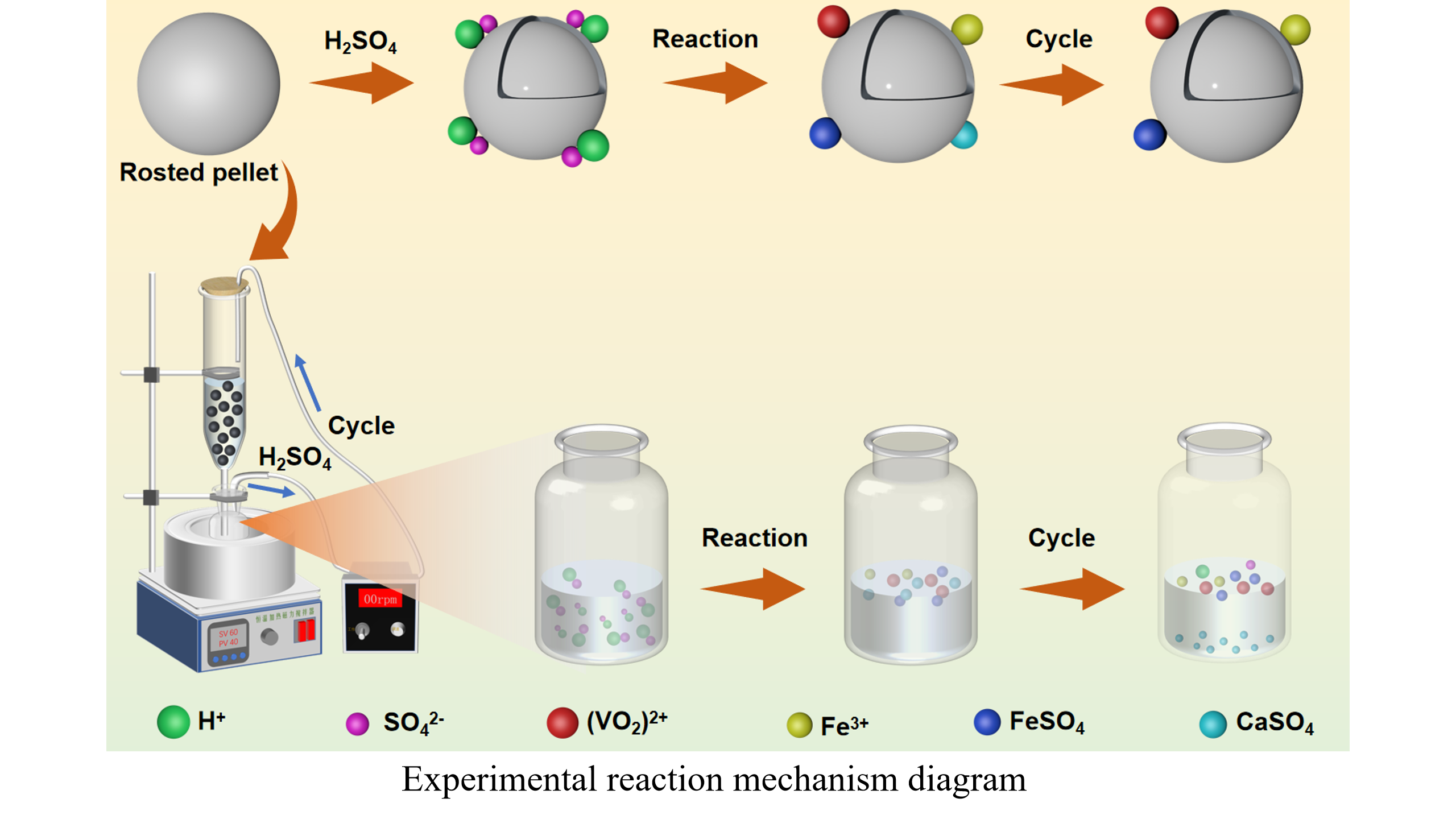
Here, a process for leaching vanadium from calcified roasting pellets (CPVC) of vanadium-titanium iron concentrate by a two-stage sulfuric acid cycle was proposed. When the silicon removal acid concentration of the pellet in the first stage was 1.5 mol/L, the solid-liquid ratio was 6: 1, the silicon removal acid concentration of the leaching solution was 3.0 mol/L, and the standing time was 48 h, the silica gel formation time was 23 h, the filtration time was 70 s, and the loss rates of vanadium and iron were 1.52% and 0.17%, respectively. When the acid concentration was 2 mol/L, at room temperature, using a leaching time of 28 days, and a solid-liquid ratio of 5: 1 in the second stage, the total leaching rates of vanadium and iron were 75.52% and 0.71%, respectively. The concentration of vanadium in the leaching solution reached 6.80 g/L, and vanadium was directly precipitated without extraction. After secondary roasting, the crushing strength of the pellets reached 2250 N, which met the requirement for blast furnace iron making. The Eh-pH diagrams of the V-Fe-H2O system at different temperatures were plotted. Thermodynamically, it was difficult to selectively leach vanadium and iron by changing the conventional acid leaching conditions. In addition, the pellets before and after leaching were analyzed. The grade of iron in the pellets increased slightly after leaching, and the main phases in the pellets remained as Fe2O3 and Fe9TiO15. The S in the sulfuric acid solution entered the leached pellets during the acid leaching reaction and was removed by the secondary roasting of the leached pellets.