Submitted:
26 June 2024
Posted:
10 July 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
| NCT Order | Disease status | NCT & Phase - Type I IFNs | NCT & Phase - Type III IFNs |
| I | COVID-19 | NCT04469491, Phase 2 (Oral/Nasal) | NCT04354259, Phase 2 (Pegylated, subcutaneous) |
| II | COVID-19 | NCT04469491, Phase 2 (Oral/Nasal) | NCT04967430, Phase 3 (Pegylated, subcutaneous) |
| III | COVID-19 | NCT04732949, Completed (Oral/Nasal) | NCT04534673, Phase 2 (Pegylated, subcutaneous) |
| IV | COVID-19 | NCT04350281, Phase 2 (Subcutaneous) | NCT04343976, Phase 2 (Pegylated, subcutaneous) |
| V | COVID-19 | NCT05381363, Phase 1/2 (Oral/Nasal) | NCT04727424, Phase 3 (Pegylated, subcutaneous) |
| VI | IAV infection | NCT00895947, Completed (Oral/Nasal) | Not available yet |
| VII | Hepatitis B/C | NCT00917358, Completed | NCT01204762, Completed (Pegylated, subcutaneous) |
| VIII | HIV Infection | NCT01295515, Completed | NCT01866930, Completed (Pegylated, subcutaneous) - HCV patients co-infected with HIV |
| IX | Cancer | NCT01462773, Completed | NCT04469491, Unknown status |
| X | Cancer | NCT00278174, Completed | Not available yet |
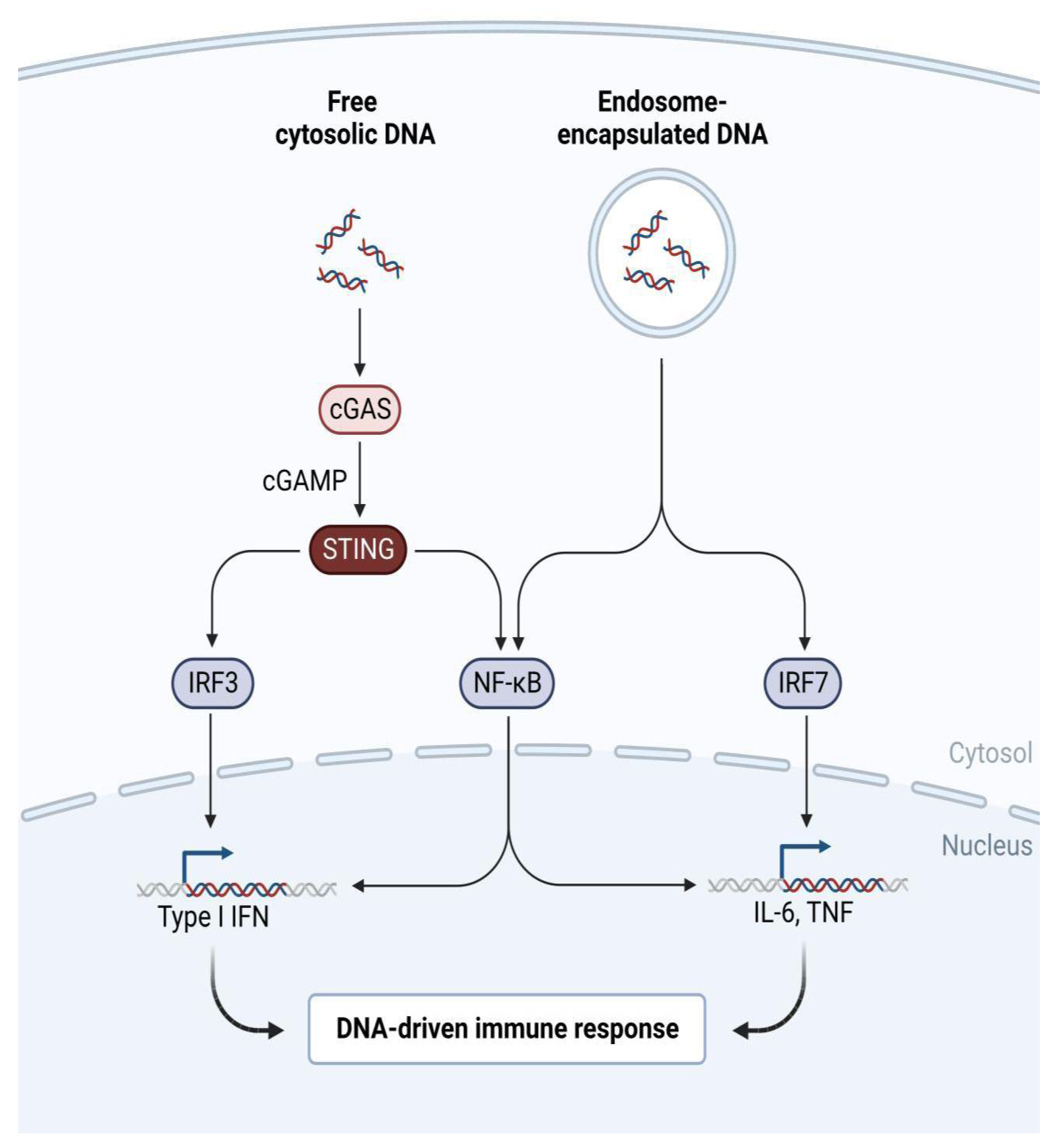
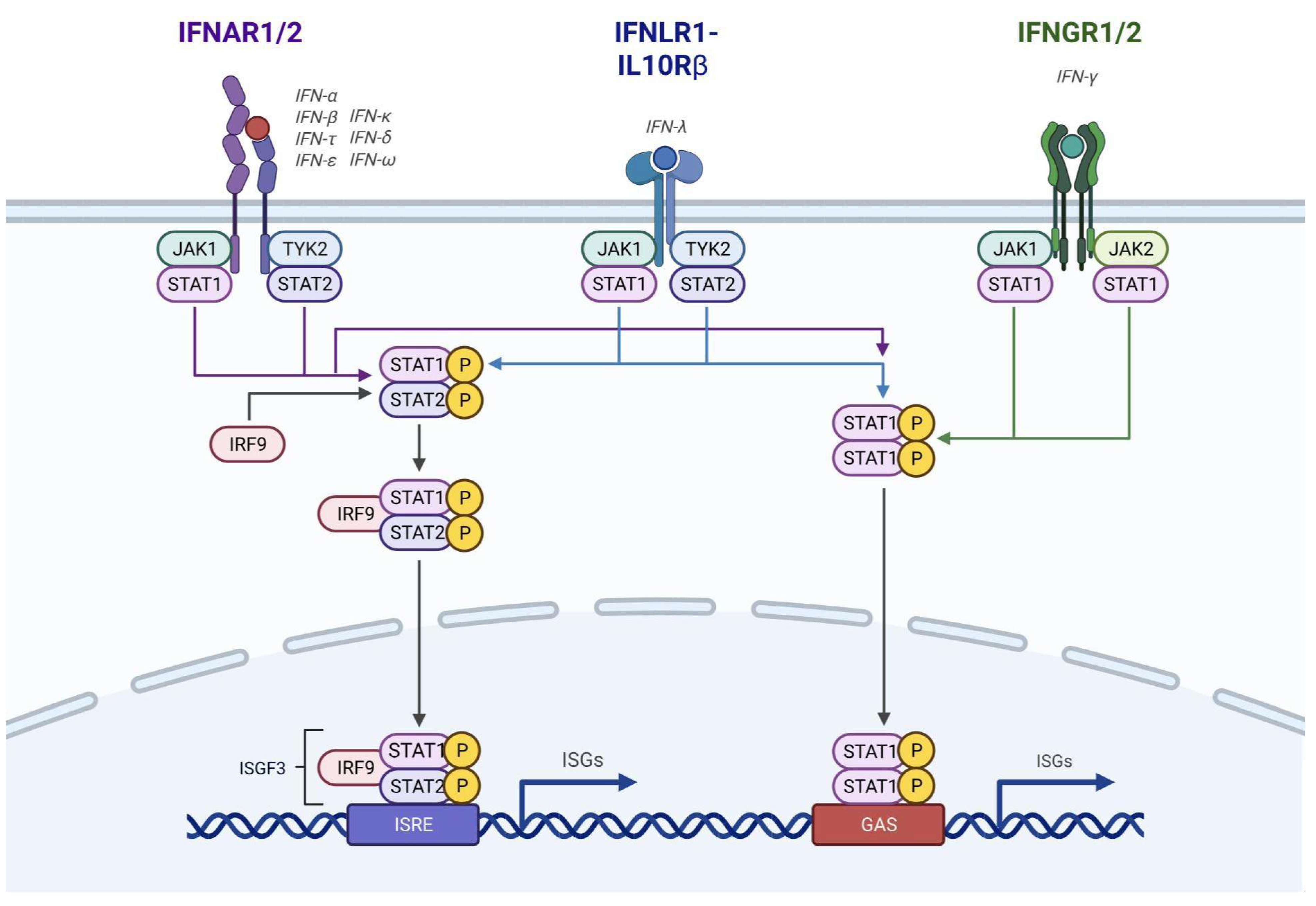
Methodology
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
List of Abbreviations
References
- Ablasser, A., Bauernfeind, F., Hartmann, G. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III–transcribed RNA intermediate. Nat Immunol 10, 1065–1072 (2009). [CrossRef]
- Ablasser, A., Hertrich, C., Waßermann, R., & Hornung, V. (2013). Nucleic acid driven sterile inflammation. Clinical immunology (Orlando, Fla.), 147(3), 207–215. [CrossRef]
- Aboulnasr, F., Hazari, S., Nayak, S., Chandra, P. K., Panigrahi, R., Ferraris, P., Chava, S., Kurt, R., Song, K., Dash, A., Balart, L. A., Garry, R. F., Wu, T., & Dash, S. (2015). IFN-λ Inhibits MiR-122 Transcription through a Stat3-HNF4α Inflammatory Feedback Loop in an IFN-α Resistant HCV Cell Culture System. PloS one, 10(12), e0141655. [CrossRef]
- Abushahba, W., Balan, M., Castaneda, I., Yuan, Y., Reuhl, K., Raveche, E., de la Torre, A., Lasfar, A., & Kotenko, S. V. (2010). Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer immunology, immunotherapy : CII, 59(7), 1059–1071. [CrossRef]
- Acevedo Ospina, H., Guay-Vincent, M.-M., & Descoteaux, A. (2022). Macrophage mitochondrial biogenesis and metabolic reprogramming induced by leishmania donovani require lipophosphoglycan and type I interferon signaling. MBio. [CrossRef]
- Acosta, P. L., Byrne, A. B., Hijano, D. R., & Talarico, L. B. (2020). Human Type I Interferon Antiviral Effects in Respiratory and Re-emerging Viral Infections. Journal of immunology research, 2020, 1372494. [CrossRef]
- Adolfo Garcia-Sastre and Christine A. Biron (2006), Type I Interferons and the Virus-Host Relationship: A lesson in Detente, Science, Vol 312, 5775, pp 879-882. [CrossRef]
- Adusumilli, N. C., Zhang, D., Friedman, J. M., & Friedman, A. J. (2020). Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric oxide : biology and chemistry, 103, 4–8. [CrossRef]
- Agy, M. B., Acker, R. L., Sherbert, C. H., & Katze, M. G. (1995). Interferon treatment inhibits virus replication in HIV-1- and SIV-infected CD4+ T-cell lines by distinct mechanisms: evidence for decreased stability and aberrant processing of HIV-1 proteins. Virology, 214(2), 379–386. [CrossRef]
- Aiman, A., Basir, S. F., & Islam, A. (2022). Interferons Horizon Therapeutics. In (Ed.), Basic and Clinical Aspects of Interferon Gamma. IntechOpen. [CrossRef]
- Akiyama, H., Ramirez, N. P., Gibson, G., Kline, C., Watkins, S., Ambrose, Z., & Gummuluru, S. (2017). Interferon-Inducible CD169/Siglec1 Attenuates Anti-HIV-1 Effects of Alpha Interferon. Journal of virology, 91(21), e00972-17. [CrossRef]
- Albrecht, J. D., Ninosu, N., Barry, D., Albrecht, T., Schaarschmidt, M. L., Goerdt, S., & Nicolay, J. P. (2022). Non-pegylated and Pegylated Interferon Alpha-2a in Cutaneous T-cell Lymphoma and the Risk of Severe Ocular Side-effects. Acta dermato-venereologica, 102, adv00722. [CrossRef]
- Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Current Issues in Molecular Biology. 2022; 44(3):1115-1126. [CrossRef]
- Alexander, M. R., Brice, A. M., Jansen van Vuren, P., Rootes, C. L., Tribolet, L., Cowled, C., Bean, A., & Stewart, C. R. (2021). Ribosome-Profiling Reveals Restricted Post Transcriptional Expression of Antiviral Cytokines and Transcription Factors during SARS-CoV-2 Infection. International journal of molecular sciences, 22(7), 3392. [CrossRef]
- Alfi, O., Yakirevitch, A., Wald, O., Wandel, O., Izhar, U., Oiknine-Djian, E., Nevo, Y., Elgavish, S., Dagan, E., Madgar, O., Feinmesser, G., Pikarsky, E., Bronstein, M., Vorontsov, O., Jonas, W., Ives, J., Walter, J., Zakay-Rones, Z., Oberbaum, M., Panet, A., … Wolf, D. G. (2021). Human Nasal and Lung Tissues Infected Ex Vivo with SARS-CoV-2 Provide Insights into Differential Tissue-Specific and Virus-Specific Innate Immune Responses in the Upper and Lower Respiratory Tract. Journal of virology, 95(14), e0013021. [CrossRef]
- Ali, S., Mann-Nüttel, R., Schulze, A., Richter, L., Alferink, J., & Scheu, S. (2019). Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver's Seat. Frontiers in immunology, 10, 778. [CrossRef]
- Alibek K, Tskhay A (2020) Ahead of a vaccine: A safe method of protection against COVID-19 exists. Research Ideas and Outcomes 6: e61709. [CrossRef]
- Alice Barbarin, André Herbelin, Jean-Marc Gombert. [The CD8+ T cell innate function in the war against cancer].. médecine/sciences, EDP Sciences, 2017, 33 (11), pp.927-929. 10.1051/med-sci/20173311004. inserm-02508042 (PDF) [The CD8+ T cell innate function in the war against cancer].. Available from: https://www.researchgate.net/publication/339946901_The_CD8_T_cell_innate_function_in_the_war_against_cancer [accessed Oct 05 2022].
- Alicea-Torres, K., Sanseviero, E., Gui, J., Chen, J., Veglia, F., Yu, Q., Donthireddy, L., Kossenkov, A., Lin, C., Fu, S., Mulligan, C., Nam, B., Masters, G., Denstman, F., Bennett, J., Hockstein, N., Rynda-Apple, A., Nefedova, Y., Fuchs, S. Y., & Gabrilovich, D. I. (2021). Immune suppressive activity of myeloid-derived suppressor cells in cancer requires inactivation of the type I interferon pathway. Nature communications, 12(1), 1717. [CrossRef]
- Aliyari, S. R., Quanquin, N., Pernet, O., Zhang, S., Wang, L., & Cheng, G. (2022). The Evolutionary Dance between Innate Host Antiviral Pathways and SARS-CoV-2. Pathogens (Basel, Switzerland), 11(5), 538. [CrossRef]
- Alunno, A., Najm, A., Mariette, X., De Marco, G., Emmel, J., Mason, L., McGonagle, D. G., & Machado, P. M. (2021). Immunomodulatory therapies for SARS-CoV-2 infection: a systematic literature review to inform EULAR points to consider. Annals of the rheumatic diseases, 80(6), 803–815. [CrossRef]
- Al-Zaidan, L., Mestiri, S., Raza, A., Merhi, M., Inchakalody, V. P., Fernandes, Q., Taib, N., Uddin, S., & Dermime, S. (2021). The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 43(1), 177–196. [CrossRef]
- Amanda J. Lee and Ali A. Ashkar (2018), The Dual Nature of Type I and Type II Interferons, Frontline Immunology. [CrossRef]
- Amanda J. Lee and Ali A. Ashkar (2018), The Dual Nature of Type I and Type II Interferons, Frontline Immunology. [CrossRef]
- Amarante-Mendes, G. P., Adjemian, S., Branco, L. M., Zanetti, L. C., Weinlich, R., & Bortoluci, K. R. (2018). Pattern recognition receptors and the host cell death molecular machinery. Frontiers in immunology, 9, 2379. [CrossRef]
- Amos C Lee, Yunjin Jeong, Sumin Lee, Haewook Jang, Allen Zheng, Sunghoon Kwon and John E. Repine (19/05/2021), Nasopharyngeal Type I-Interferon for Immediately Available Prophylaxis Against Emerging Respiratory Viral Infections, Front. Immunol., 12.660298. [CrossRef]
- Andersson, U., Yang, H., & Harris, H. (2018). High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Seminars in immunology, 38, 40–48. [CrossRef]
- Anjum, F. R., Anam, S., Abbas, G., Mahmood, M. S., Rahman, S. U., Goraya, M. U., Abdullah, R. M., Luqman, M., Ali, A., Akram, M. K., & Chaudhry, T. H. (2021). Type I IFNs: A Blessing in Disguise or Partner in Crime in MERS-CoV-, SARS-CoV-, and SARS-CoV-2-Induced Pathology and Potential Use of Type I IFNs in Synergism with IFN-γ as a Novel Antiviral Approach Against COVID-19. Viral immunology, 34(5), 321–329. [CrossRef]
- Ank, N., West, H., Bartholdy, C., Eriksson, K., Thomsen, A. R., & Paludan, S. R. (2006). Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. Journal of virology, 80(9), 4501–4509. [CrossRef]
- Arndt, W. D., Cotsmire, S., Trainor, K., Harrington, H., Hauns, K., Kibler, K. V., Huynh, T. P., & Jacobs, B. L. (2015). Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus. Journal of virology, 89(20), 10489–10499. [CrossRef]
- Arvin, A. M., Fink, K., Schmid, M. A., Cathcart, A., Spreafico, R., Havenar-Daughton, C., Lanzavecchia, A., Corti, D., & Virgin, H. W. (2020). A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature, 584(7821), 353–363. [CrossRef]
- Avota, E., Gassert, E., & Schneider-Schaulies, S. (2010). Measles virus-induced immunosuppression: from effectors to mechanisms. Medical microbiology and immunology, 199(3), 227–237. [CrossRef]
- Avota, E., Koethe, S., & Schneider-Schaulies, S. (2013). Membrane dynamics and interactions in measles virus dendritic cell infections. Cellular microbiology, 15(2), 161–169. [CrossRef]
- Azamor, T., Cunha, D. P., da Silva, A., Bezerra, O., Ribeiro-Alves, M., Calvo, T. L., Kehdy, F., Manta, F., Pinto, T., Ferreira, L. P., Portari, E. A., Guida, L., Gomes, L., Moreira, M., de Carvalho, E. F., Cardoso, C. C., Muller, M., Ano Bom, A., Neves, P., Vasconcelos, Z., … Moraes, M. O. (2021). Congenital Zika Syndrome Is Associated With Interferon Alfa Receptor 1. Frontiers in immunology, 12, 764746. [CrossRef]
- Babiuch, L., Mian, M., Kamińska, E., Szymańska, B., & Georgiades, J. A. (1993). An interim report on the effect of natural human interferon alpha (IFN-alpha) lozenges in patients seropositive for the human immunodeficiency virus type 1 (HIV-1). Archivum immunologiae et therapiae experimentalis, 41(3-4), 213–219, doi: https://pubmed.ncbi.nlm.nih.gov/7907465/.
- Baechler, E. C., Bilgic, H., & Reed, A. M. (2011). Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis research & therapy, 13(6), 249. [CrossRef]
- BAI, H., KAWAHARA, M., TAKAHASHI, M., & IMAKAWA, K. (2022). Recent progress of Interferon-Tau Research and potential direction beyond pregnancy recognition. Journal of Reproduction and Development, 68(5), 299–306. [CrossRef]
- Barber G. N. (2001). Host defense, viruses and apoptosis. Cell death and differentiation, 8(2), 113–126. [CrossRef]
- Barilli, A., Visigalli, R., Ferrari, F., Recchia Luciani, G., Soli, M., Dall'Asta, V., & Rotoli, B. M. (2022). The JAK1/2 Inhibitor Baricitinib Mitigates the Spike-Induced Inflammatory Response of Immune and Endothelial Cells In Vitro. Biomedicines, 10(9), 2324. [CrossRef]
- Barros-Martins, J., Förster, R. & Bošnjak, B. NK cell dysfunction in severe COVID-19: TGF-β-induced downregulation of integrin beta-2 restricts NK cell cytotoxicity. Sig Transduct Target Ther 7, 32 (2022). [CrossRef]
- Baruch, K., Deczkowska, A., David, E., Castellano, J. M., Miller, O., Kertser, A., Berkutzki, T., Barnett-Itzhaki, Z., Bezalel, D., Wyss-Coray, T., Amit, I., & Schwartz, M. (2014). Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science (New York, N.Y.), 346(6205), 89–93. [CrossRef]
- Basler C. F. (2012). Nipah and hendra virus interactions with the innate immune system. Current topics in microbiology and immunology, 359, 123–152. [CrossRef]
- Basler C. F. (2015). Innate immune evasion by filoviruses. Virology, 479-480, 122–130. [CrossRef]
- Basler, C. F., & Amarasinghe, G. K. (2009). Evasion of interferon responses by Ebola and Marburg viruses. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 29(9), 511–520. [CrossRef]
- Bastard, P., Rosen, L. B., Zhang, Q., Michailidis, E., Hoffmann, H. H., Zhang, Y., Dorgham, K., Philippot, Q., Rosain, J., Béziat, V., Manry, J., Shaw, E., Haljasmägi, L., Peterson, P., Lorenzo, L., Bizien, L., Trouillet-Assant, S., Dobbs, K., de Jesus, A. A., Belot, A., … Casanova, J. L. (2020). Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, N.Y.), 370(6515), eabd4585. [CrossRef]
- Batiha, G. E., Al-Gareeb, D., Qusti, S., Alshammari, E. M., Rotimi, D., Adeyemi, O. S., & Al-Kuraishy, H. M. (2021). Common NLRP3 inflammasome inhibitors and Covid- 19. [CrossRef]
- Battistini, C., Ballan, R., Herkenhoff, M. E., Saad, S., & Sun, J. (2020). Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. International journal of molecular sciences, 22(1), 362. [CrossRef]
- Bayati, A., Kumar, R., Francis, V., & McPherson, P. S. (2021). SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. The Journal of biological chemistry, 296, 100306. [CrossRef]
- Beldarraín, A., Cruz, Y., Cruz, O., Navarro, M. and Gil, M., 2001. Purification and conformational properties of a human interferon α2b produced in Escherichia coli. Biotechnology and applied biochemistry, 33(3), pp.173-182. [CrossRef]
- Bellanti, F., Vendemiale, G., Altomare, E., & Serviddio, G. (2012). The impact of interferon lambda 3 gene polymorphism on natural course and treatment of hepatitis C. Clinical & developmental immunology, 2012, 849373. [CrossRef]
- Bennett, A. L., Smith, D. W., Cummins, M. J., Jacoby, P. A., Cummins, J. M., & Beilharz, M. W. (2013). Low-dose oral interferon alpha as prophylaxis against viral respiratory illness: a double-blind, parallel controlled trial during an influenza pandemic year. Influenza and other respiratory viruses, 7(5), 854–862. [CrossRef]
- Bermejo-Jambrina, M., Blatzer, M., Jauregui-Onieva, P., Yordanov, T. E., Hörtnagl, P., Valovka, T., Huber, L. A., Wilflingseder, D., & Posch, W. (2020). CR4 Signaling Contributes to a DC-Driven Enhanced Immune Response Against Complement-Opsonized HIV-1. Frontiers in immunology, 11, 2010. [CrossRef]
- Bharaj, P. , Wang, Y. E., Dawes, B. E., Yun, T. E., Park, A., Yen, B., Basler, C. F., Freiberg, A. N., Lee, B., & Rajsbaum, R. (2016). The Matrix Protein of Nipah Virus Targets the E3-Ubiquitin Ligase TRIM6 to Inhibit the IKKε Kinase-Mediated Type-I IFN Antiviral Response. PLoS pathogens, 12(9), e1005880. [CrossRef]
- Bian, Q., Lu, J., Zhang, L., Chi, Y., Li, Y., & Guo, H. (2017). Highly pathogenic avian influenza A virus H5N1 non-structural protein 1 is associated with apoptotic activation of the intrinsic mitochondrial pathway. Experimental and therapeutic medicine, 14(5), 4041–4046. [CrossRef]
- Bigay, J., Le Grand, R., Martinon, F., & Maisonnasse, P. (2022). Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Frontiers in microbiology, 13, 932408. [CrossRef]
- Birkhoff, M., Leitz, M., & Marx, D. (2009). Advantages of Intranasal Vaccination and Considerations on Device Selection. Indian Journal of Pharmaceutical Sciences, 71(6), 729–731.
- Birkhoff, M. Birkhoff, M., Leitz, M., & Marx, D. (2009). Advantages of Intranasal Vaccination and Considerations on Device Selection. Indian Journal of Pharmaceutical Sciences, 71(6), 729–731.
- Biron, C. A. (2016, February 12). Innate immunity: Recognizing and responding to foreign invaders-no training needed. Viral Pathogenesis (Third Edition). Retrieved October 22, 2022, from https://www.sciencedirect.com/science/article/pii/B9780128009642000045.
- Bittner, Z. A., Schrader, M., George, S. E., & Amann, R. (2022). Pyroptosis and Its Role in SARS-CoV-2 Infection. Cells, 11(10), 1717. [CrossRef]
- Blank, T., & Prinz, M. (2017). Type I interferon pathway in CNS homeostasis and neurological disorders. Glia, 65(9), 1397–1406. [CrossRef]
- Block, J. (2021). Vaccinating people who have had covid-19: why doesn’t natural immunity count in the US?. BMJ, n2101. [CrossRef]
- Bo, Z., Miao, Y., Xi, R., Zhong, Q., Bao, C., Chen, H., Sun, L., Qian, Y., Jung, Y. S., & Dai, J. (2020). PRV UL13 inhibits cGAS-STING-mediated IFN-β production by phosphorylating IRF3. Veterinary research, 51(1), 118. [CrossRef]
- Booth, L., Roberts, J. L., Ecroyd, H., Tritsch, S. R., Bavari, S., Reid, S. P., Proniuk, S., Zukiwski, A., Jacob, A., Sepúlveda, C. S., Giovannoni, F., García, C. C., Damonte, E., González-Gallego, J., Tuñón, M. J., & Dent, P. (2016). AR-12 Inhibits Multiple Chaperones Concomitant With Stimulating Autophagosome Formation Collectively Preventing Virus Replication. Journal of cellular physiology, 231(10), 2286–2302. [CrossRef]
- Borden, E.C. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov 18, 219–234 (2019). [CrossRef]
- Botek, M., Krejčí, J., Valenta, M., McKune, A., Sládečková, B., Konečný, P., Klimešová, I., & Pastucha, D. (2022). Molecular Hydrogen Positively Affects Physical and Respiratory Function in Acute Post-COVID-19 Patients: A New Perspective in Rehabilitation. International journal of environmental research and public health, 19(4), 1992. [CrossRef]
- Bournazos, S., Gupta, A., & Ravetch, J. V. (2020). The role of IgG Fc receptors in antibody-dependent enhancement. Nature reviews. Immunology, 20(10), 633–643. [CrossRef]
- Bousse, T., Chambers, R. L., Scroggs, R. A., Portner, A., & Takimoto, T. (2006). Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus research, 121(1), 23–32. [CrossRef]
- Bowie, A. G., & Unterholzner, L. (2008). Viral evasion and subversion of pattern-recognition receptor signalling. Nature reviews. Immunology, 8(12), 911–922. [CrossRef]
- Bracci, L., La Sorsa, V., Belardelli, F., & Proietti, E. (2008). Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert review of vaccines, 7(3), 373–381. [CrossRef]
- Bressy, C., Droby, G. N., Maldonado, B. D., Steuerwald, N., & Grdzelishvili, V. Z. (2019). Cell Cycle Arrest in G2/M Phase Enhances Replication of Interferon-Sensitive Cytoplasmic RNA Viruses via Inhibition of Antiviral Gene Expression. Journal of virology, 93(4), e01885-18. [CrossRef]
- Brisse, M., & Ly, H. (2019). Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Frontiers in immunology, 10, 1586. [CrossRef]
- Brochet B. (2008). Activité à long terme de l'acétate de glatiramère dans le traitement de la sclérose en plaques : état des connaissances [Long-term effects of glatiramer acetate in multiple sclerosis]. Revue neurologique, 164(11), 917–926. [CrossRef]
- Brown, M., & Bhardwaj, N. (2021). Super(antigen) target for SARS-CoV-2. Nature reviews. Immunology, 21(2), 72. [CrossRef]
- Brown, M., & Bhardwaj, N. (2021). Super(antigen) target for SARS-CoV-2. Nature reviews. Immunology, 21(2), 72. [CrossRef]
- Bruttel, V., Washburne, A., & VanDongen, A. (2022). Endonuclease fingerprint indicates a synthetic origin of SARS-COV-2. [CrossRef]
- Brzózka, K., Finke, S., & Conzelmann, K. K. (2005). Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. Journal of virology, 79(12), 7673–7681. [CrossRef]
- Burns K. H. (2020). Our Conflict with Transposable Elements and Its Implications for Human Disease. Annual review of pathology, 15, 51–70. [CrossRef]
- Busnadiego, I., Fernbach, S., Pohl, M. O., Karakus, U., Huber, M., Trkola, A., Stertz, S., & Hale, B. G. (2020). Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio, 11(5), e01928-20. [CrossRef]
- Busnadiego, I., Fernbach, S., Pohl, M. O., Karakus, U., Huber, M., Trkola, A., Stertz, S., & Hale, B. G. (2020). Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio, 11(5), e01928-20. [CrossRef]
- Busnadiego, I., Fernbach, S., Pohl, M. O., Karakus, U., Huber, M., Trkola, A., Stertz, S., & Hale, B. G. (2020). Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio, 11(5), e01928-20. [CrossRef]
- Buzhdygan, T. P., DeOre, B. J., Baldwin-Leclair, A., Bullock, T. A., McGary, H. M., Khan, J. A., Razmpour, R., Hale, J. F., Galie, P. A., Potula, R., Andrews, A. M., & Ramirez, S. H. (2020). The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiology of disease, 146, 105131. [CrossRef]
- Cabanillas, B., Akdis, C., & Novak, N. (2021). Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol?. Allergy, 76(6), 1617-1618. [CrossRef]
- Cadegiani F. A. (2022). Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings. Cureus, 14(8), e27883. [CrossRef]
- Can Li, Yanxia Chen, Yan Zhao, David Christopher Lung, Zhanhong Ye, Wenchen Song, Fei-Fei Liu, Jian-Piao Cai, Wan-Man Wong, Cyril Chik-Yan Yip, Jasper Fuk-Woo Chan, Kelvin Kai-Wang To, Siddharth Sridhar, Ivan Fan-Ngai Hung, Hin Chu, Kin-Hang Kok, Dong-Yan Jin, Anna Jinxia Zhang, Kwok-Yung Yuen, Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model, Clinical Infectious Diseases, 2021;, ciab707. [CrossRef]
- Cao, L., Ge, X., Gao, Y., Herrler, G., Ren, Y., Ren, X., & Li, G. (2015). Porcine epidemic diarrhea virus inhibits dsRNA-induced interferon-β production in porcine intestinal epithelial cells by blockade of the RIG-I-mediated pathway. Virology journal, 12, 127. [CrossRef]
- Carella, C., Mazziotti, G., Morisco, F., Manganella, G., Rotondi, M., Tuccillo, C., Sorvillo, F., Caporaso, N., & Amato, G. (2001). Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. The Journal of clinical endocrinology and metabolism, 86(5), 1925–1929. [CrossRef]
- Carlos, A. J., Ha, D. P., Yeh, D. W., Van Krieken, R., Tseng, C. C., Zhang, P., Gill, P., Machida, K., & Lee, A. S. (2021). The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. The Journal of biological chemistry, 296, 100759. [CrossRef]
- Carp, T. N. (2024). How the Next Pandemic Could Be Handled— Studying the Risks of (A)H5N1 Influenza Human Spillover. Preprints. [CrossRef]
- Carp, T. N. (2024). How the Next Pandemic Could Be Handled— Studying the Risks of (A)H5N1 Influenza Human Spillover. Preprints. [CrossRef]
- Carrero J. A. (2013). Confounding roles for type I interferons during bacterial and viral pathogenesis. International immunology, 25(12), 663–669. [CrossRef]
- Carvajal Ibañez, D., Skabkin, M., Hooli, J., Cerrizuela, S., Göpferich, M., Jolly, A.,... & Martin-Villalba, A. (2023). Interferon regulates neural stem cell function at all ages by orchestrating mTOR and cell cycle. EMBO Molecular Medicine, e16434. [CrossRef]
- Case, J. B., Ashbrook, A. W., Dermody, T. S., & Denison, M. R. (2016). Mutagenesis of S-Adenosyl-l-Methionine-Binding Residues in Coronavirus nsp14 N7-Methyltransferase Demonstrates Differing Requirements for Genome Translation and Resistance to Innate Immunity. Journal of virology, 90(16), 7248–7256. [CrossRef]
- Cassius, C., Amode, R., Delord, M., Battistella, M., Poirot, J., How-Kit, A., Lepelletier, C., Jachiet, M., de Masson, A., Frumholtz, L., Cordoliani, F., Boccara, D., Lehmann-Che, J., Wong, J., Dubanchet, S., Alberdi, A. J., Merandet, M., Bagot, M., Bensussan, A., Bouaziz, J. D., … Le Buanec, H. (2020). MDA5+ Dermatomyositis Is Associated with Stronger Skin Type I Interferon Transcriptomic Signature with Upregulation of IFN-κ Transcript. The Journal of investigative dermatology, 140(6), 1276–1279.e7. [CrossRef]
- Casteleyn, C., Broos, A. M., Simoens, P., & Van den Broeck, W. (2010). NALT (nasal cavity-associated lymphoid tissue) in the rabbit. Veterinary immunology and immunopathology, 133(2-4), 212–218. [CrossRef]
- Castro, L.S., Lobo, G.S., Pereira, P., Freire, M.G., Neves, M.C. and Pedro, A.Q., 2021. Interferon-based biopharmaceuticals: Overview on the production, purification, and formulation. Vaccines, 9(4), p.328. [CrossRef]
- Castro-Jiménez, T. K., Gómez-Legorreta, L. C., López-Campa, L. A., Martínez-Torres, V., Alvarado-Silva, M., Posadas-Mondragón, A., Díaz-Lima, N., Angulo-Mendez, H. A., Mejía-Domínguez, N. R., Vaca-Paniagua, F., Ávila-Moreno, F., García-Cordero, J., Cedillo-Barrón, L., Aguilar-Ruíz, S. R., & Bustos-Arriaga, J. (2022). Variability in Susceptibility to Type I Interferon Response and Subgenomic RNA Accumulation Between Clinical Isolates of Dengue and Zika Virus From Oaxaca Mexico Correlate With Replication Efficiency in Human Cells and Disease Severity. Frontiers in cellular and infection microbiology, 12, 890750. [CrossRef]
- Cataldi, M., Shah, N. R., Felt, S. A., & Grdzelishvili, V. Z. (2015). Breaking resistance of pancreatic cancer cells to an attenuated vesicular stomatitis virus through a novel activity of IKK inhibitor TPCA-1. Virology, 485, 340–354. [CrossRef]
- Cerezo, L., & Macià I Garau, M. (2012). Acute radiation syndrome and Fukushima: A watershed moment?. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology, 17(1), 1–3. [CrossRef]
- Cerpa-Cruz, S., Paredes-Casillas, P., Landeros Navarro, E. et al. Adverse events following immunization with vaccines containing adjuvants. Immunol Res 56, 299–303 (2013). [CrossRef]
- Cervantes, J., Weinerman, B., Basole, C. et al. TLR8: the forgotten relative revindicated. Cell Mol Immunol 9, 434–438 (2012). [CrossRef]
- Chan, R., Chan, K., Lui, G., Tsun, J., Chan, K., Yip, J., Liu, S., Yu, M., Ng, R., Chong, K., Wang, M. H., Chan, P., Li, A. M., & Lam, H. S. (2022). Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study. Pathogens (Basel, Switzerland), 11(4), 397. [CrossRef]
- Chattree, V., Singh, K., Singh, K., Goel, A., Maity, A., & Lone, A. (2022). A comprehensive review on modulation of SIRT1 signaling pathways in the immune system of COVID-19 patients by phytotherapeutic melatonin and epigallocatechin-3-gallate. Journal of food biochemistry, e14259. Advance online publication. [CrossRef]
- Chauss, D., Freiwald, T., McGregor, R., Yan, B., Wang, L., Nova-Lamperti, E., Kumar, D., Zhang, Z., Teague, H., West, E. E., Vannella, K. M., Ramos-Benitez, M. J., Bibby, J., Kelly, A., Malik, A., Freeman, A. F., Schwartz, D. M., Portilla, D., Chertow, D. S., John, S., … Afzali, B. (2022). Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nature immunology, 23(1), 62–74. [CrossRef]
- Chen, A. et al. (2021) “Chitinase-3-like 1 protein complexes modulate macrophage-mediated immune suppression in glioblastoma,” Journal of Clinical Investigation, 131(16). [CrossRef]
- Chen, K., Liu, J., & Cao, X. (2017). Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. Journal of autoimmunity, 83, 1–11. [CrossRef]
- Chen, X., Jiang, L., & Liu, X. (2022). Natural killer cells: the next wave in cancer immunotherapy. Frontiers in immunology, 13, 954804. [CrossRef]
- Chen, Y., & Colonna, M. (2021). Microglia in Alzheimer's disease at single-cell level. Are there common patterns in humans and mice? The Journal of experimental medicine, 218(9), e20202717. [CrossRef]
- Chieux, V., Hober, D., Chehadeh, W., & Wattré, P. (1999). Interféron alpha, protéines antivirales et applications médicales [Alpha interferon, antiviral proteins and their value in clinical medicine]. Annales de biologie clinique, 57(6), 659–666.
- Childs, B., & van Deursen, J. (2019). Inhibition of 'jumping genes' promotes healthy ageing. Nature, 566(7742), 46–48. [CrossRef]
- Cho, C., , Mukherjee, R., Peck, A. R., Sun, Y., McBrearty, N., Katlinski, K. V., Gui, J., Govindaraju, P. K., Puré, E., Rui, H., & Fuchs, S. Y. (2020). Cancer-associated fibroblasts downregulate type I interferon receptor to stimulate intratumoral stromagenesis. Oncogene, 39(38), 6129–6137. [CrossRef]
- Cho, H. J., Hwang, J. A., Yang, E. J., Kim, E. C., Kim, J. R., Kim, S. Y., Kim, Y. Z., Park, S. C., & Lee, Y. S. (2022). Nintedanib induces senolytic effect via STAT3 inhibition. Cell death & disease, 13(9), 760. [CrossRef]
- Choi, H., & Shin, E. C. (2021). Roles of Type I and III Interferons in COVID-19. Yonsei medical journal, 62(5), 381–390. [CrossRef]
- Choo, O.-S., Lee, Y. Y., Kim, Y. S., Kim, Y. J., Lee, D. H., Kim, H., Jang, J. H., & Choung, Y.-H. (2022). Effect of statin on age-related hearing loss via drug repurposing. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research, 1869(11), 119331. [CrossRef]
- Choudhary, A. (2021). Immune regulatory function of interferon-gamma in acute leukemia. INDIAN JOURNAL OF APPLIED RESEARCH, 75–77. [CrossRef]
- Chung, J. H., Hong, S. H., Seo, N., Kim, T. S., An, H. J., Lee, P., Shin, E. C., & Kim, H. M. (2020). Structure-based glycoengineering of interferon lambda 4 enhances its productivity and anti-viral potency. Cytokine, 125, 154833. [CrossRef]
- Civril, F., Deimling, T., de Oliveira Mann, C. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013). [CrossRef]
- Claudio G., Gallo, Sirio Fiorino, Giovanni Posabella, Donato Antonacci, Antonio Tropeano, Emanuele Pausini, Carlotta Pausini, Tomasso Guarniero, Marco Zancanaro (2020), COVID-19.
- Corona, A., Fanunza, E., Salata, C., Morwitzer, M. J., Distinto, S., Zinzula, L., Sanna, C., Frau, A., Daino, G. L., Quartu, M., Taglialatela-Scafati, O., Rigano, D., Reid, S., Mirazimi, A., & Tramontano, E. (2022). Cynarin blocks Ebola virus replication by counteracting VP35 inhibition of interferon-beta production. Antiviral research, 198, 105251. [CrossRef]
- Corrales, L., McWhirter, S. M., Dubensky, T. W., Jr, & Gajewski, T. F. (2016). The host STING pathway at the interface of cancer and immunity. The Journal of clinical investigation, 126(7), 2404–2411. [CrossRef]
- Costa Silva, R., Bandeira-Melo, C., Paula Neto, H. A., Vale, A. M., & Travassos, L. H. (2022). COVID-19 diverse outcomes: Aggravated reinfection, type I interferons and antibodies. Medical hypotheses, 167, 110943. [CrossRef]
- Crow M. K. (2010). Long interspersed nuclear elements (LINE-1): potential triggers of systemic autoimmune disease. Autoimmunity, 43(1), 7–16. [CrossRef]
- Csépány, T. Csépány, T., & Bereczki, D. (2004). Immunmoduláns kezelés sclerosis multiplexben [Immunomodulatory therapy in multiple sclerosis]. Ideggyogyaszati szemle.
- Dacia Plant (2021), Interferonate tablets, doi: https://www.daciaplant.ro/interferonat-comprimate.html.
- Dagenais A, Villalba-Guerrero C and Olivier M (2023) Trained immunity: A “new” weapon in the fight against infectious diseases. Front. Immunol. 14:1147476. [CrossRef] [PubMed]
- Daniel B. Stetson and Rusian Medzhitov (2006), Type I Interferons in Host Defense, Immunity, Vol 25, pp 373-381. [CrossRef]
- Das, A., Dinh, P. X., Panda, D., & Pattnaik, A. K. (2014). Interferon-inducible protein IFI35 negatively regulates RIG-I antiviral signaling and supports vesicular stomatitis virus replication. Journal of virology, 88(6), 3103–3113. [CrossRef]
- Dasgupta, A. , Tsay, E., Federman, N., Lechner, M. G., & Su, M. A. (2022). Polyendocrine Autoimmunity and Diabetic Ketoacidosis Following Anti-PD-1 and Interferon α. Pediatrics, 149(4), e2021053363. [CrossRef]
- David Vremec, Meredith O'Keeffe, Hubertus Hochrein, Martina Fuchsberger, Irina Caminschi, Mireille Lahoud, Ken Shortman; Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood 2007; 109 (3): 1165–1173. doi:. [CrossRef]
- David Vremec, Meredith O'Keeffe, Hubertus Hochrein, Martina Fuchsberger, Irina Caminschi, Mireille Lahoud, Ken Shortman; Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood 2007; 109 (3): 1165–1173. doi:. [CrossRef]
- Davies, K. A., Cooper, E., Voon, V., Tibble, J., Cercignani, M., & Harrison, N. A. (2021). Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Molecular psychiatry, 26(9), 5150–5160. [CrossRef]
- Davoudi-Monfared, E., Rahmani, H., Khalili, H., Hajiabdolbaghi, M., Salehi, M., Abbasian, L., Kazemzadeh, H., & Yekaninejad, M. S. (2020). A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19. Antimicrobial agents and chemotherapy, 64(9), e01061-20. [CrossRef]
- Daza-Cajigal, V., Albuquerque, A. S., Young, D. F., Ciancanelli, M. J., Moulding, D., Angulo, I., Jeanne-Julien, V., Rosain, J., Minskaia, E., Casanova, J. L., Boisson-Dupuis, S., Bustamante, J., Randall, R. E., McHugh, T. D., Thrasher, A. J., & Burns, S. O. (2022). Partial human Janus kinase 1 deficiency predominantly impairs responses to interferon gamma and intracellular control of mycobacteria. Frontiers in immunology, 13, 888427. [CrossRef]
- De Cecco, M., Ito, T., Petrashen, A. P., Elias, A. E., Skvir, N. J., Criscione, S. W., Caligiana, A., Brocculi, G., Adney, E. M., Boeke, J. D., Le, O., Beauséjour, C., Ambati, J., Ambati, K., Simon, M., Seluanov, A., Gorbunova, V., Slagboom, P. E., Helfand, S. L., Neretti, N., … Sedivy, J. M. (2019). L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature, 566(7742), 73–78. [CrossRef]
- De Cecco, M., Ito, T., Petrashen, A.P. et al. Author Correction: L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 572, E5 (2019). [CrossRef]
- De Clercq, E., & Neyts, J. (2007). Avian influenza A (H5N1) infection: targets and strategies for chemotherapeutic intervention. Trends in pharmacological sciences, 28(6), 280–285. [CrossRef]
- de Groen, R. A., Groothuismink, Z. M., Liu, B. S., & Boonstra, A. (2015). IFN-λ is able to augment TLR-mediated activation and subsequent function of primary human B cells. Journal of leukocyte biology, 98(4), 623–630. [CrossRef]
- De Maeyer, E., & De Maeyer-Guignard, J. (1998). Type I interferons. International reviews of immunology, 17(1-4), 53–73. [CrossRef]
- de Witte, L. , Abt, M., Schneider-Schaulies, S., van Kooyk, Y., & Geijtenbeek, T. B. (2006). Measles virus targets DC-SIGN to enhance dendritic cell infection. Journal of virology, 80(7), 3477–3486. [CrossRef]
- Deczkowska, A., Baruch, K., & Schwartz, M. (2016). Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends in immunology, 37(3), 181–192. [CrossRef]
- DeDiego, M. L., Nogales, A., Lambert-Emo, K., Martinez-Sobrido, L., & Topham, D. J. (2016). NS1 Protein Mutation I64T Affects Interferon Responses and Virulence of Circulating H3N2 Human Influenza A Viruses. Journal of virology, 90(21), 9693–9711. [CrossRef]
- Dempoya, J., Matsumiya, T., Imaizumi, T., Hayakari, R., Xing, F., Yoshida, H., Okumura, K., & Satoh, K. (2012). Double-stranded RNA induces biphasic STAT1 phosphorylation by both type I interferon (IFN)-dependent and type I IFN-independent pathways. Journal of virology, 86(23), 12760–12769. [CrossRef]
- Deng, X. , Buckley, A. C., Pillatzki, A., Lager, K. M., Faaberg, K. S., & Baker, S. C. (2020). Inactivating Three Interferon Antagonists Attenuates Pathogenesis of an Enteric Coronavirus. Journal of virology, 94(17), e00565-20. [CrossRef]
- Desfarges, S. , Ciuffi, A. (2012). Viral Integration and Consequences on Host Gene Expression. In: Witzany, G. (eds) Viruses: Essential Agents of Life. Springer, Dordrecht. [CrossRef]
- Di Martino, J.S., Nobre, A.R., Mondal, C. et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer 3, 90–107 (2022). [CrossRef]
- Di Palma, F., Daino, G. L., Ramaswamy, V. K., Corona, A., Frau, A., Fanunza, E., Vargiu, A. V., Tramontano, E., & Ruggerone, P. (2019). Relevance of Ebola virus VP35 homo-dimerization on the type I interferon cascade inhibition. Antiviral chemistry & chemotherapy, 27, 2040206619889220. [CrossRef]
- Di Trolio, R. Di Trolio, R., Simeone, E., Di Lorenzo, G., Grimaldi, A. M., Romano, A., Ayala, F., Caracò, C., Mozzillo, N., & Ascierto, P. A. (2012). Update on PEG-interferon α-2b as adjuvant therapy in melanoma. Anticancer research, 3909. [Google Scholar]
- Diana A. Pippig, Johannes C. Hellmuth, Sheng Cui, Axel Kirchhofer, Katja Lammens, Alfred Lammens, Andreas Schmidt, Simon Rothenfusser, Karl-Peter Hopfner, The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA, Nucleic Acids Research, Volume 37, Issue 6, 1 April 2009, Pages 2014–2025. [CrossRef]
- Dick, A., Graf, L., Olal, D., von der Malsburg, A., Gao, S., Kochs, G., & Daumke, O. (2015). Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. The Journal of biological chemistry, 290(20), 12779–12792. [CrossRef]
- Didangelos A. (2020). COVID-19 Hyperinflammation: What about Neutrophils? mSphere, 5(3), e00367-20. [CrossRef]
- Ding, F., Yin, Z., & Wang, H. R. (2011). Ubiquitination in Rho signaling. Current topics in medicinal chemistry, 11(23), 2879–2887. [CrossRef]
- Ding, J., Aldo, P., Roberts, C. M., Stabach, P., Liu, H., You, Y., Qiu, X., Jeong, J., Maxwell, A., Lindenbach, B., Braddock, D., Liao, A., & Mor, G. (2021). Placenta-derived interferon-stimulated gene 20 controls ZIKA virus infection. EMBO reports, 22(10), e52450. [CrossRef]
- Dipasquale, O. , Cooper, E. A., Tibble, J., Voon, V., Baglio, F., Baselli, G., Cercignani, M., & Harrison, N. A. (2016). Interferon-α acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain, behavior, and immunity, 58, 31–39. [CrossRef]
- Doerfler W. (2021). Adenoviral Vector DNA- and SARS-CoV-2 mRNA-Based Covid-19 Vaccines: Possible Integration into the Human Genome - Are Adenoviral Genes Expressed in Vector-based Vaccines? Virus research, 302, 198466. [CrossRef]
- Dohlman, A. B., Klug, J., Mesko, M., Gao, I. H., Lipkin, S. M., Shen, X., & Iliev, I. D. (2022). A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell, 185(20), 3807–3822. [CrossRef]
- Doi, A., Iijima, K., Kano, S., & Ishizaka, Y. (2015). Viral protein R of HIV type-1 induces retrotransposition and upregulates glutamate synthesis by the signal transducer and activator of transcription 1 signaling pathway. Microbiology and immunology, 59(7), 398–409. [CrossRef]
- Dong, L. W., Kong, X. N., Yan, H. X., Yu, L. X., Chen, L., Yang, W., Liu, Q., Huang, D. D., Wu, M. C., & Wang, H. Y. (2008). Signal regulatory protein alpha negatively regulates both TLR3 and cytoplasmic pathways in type I interferon induction. Molecular immunology, 45(11), 3025–3035. [CrossRef]
- Durmaz, V., Köchl, K., Krassnigg, A., Parigger, L., Hetmann, M., Singh, A., Nutz, D., Korsunsky, A., Kahler, U., König, C., Chang, L., Krebs, M., Bassetto, R., Pavkov-Keller, T., Resch, V., Gruber, K., Steinkellner, G., & Gruber, C. C. (2022). Structural bioinformatics analysis of SARS-CoV-2 variants reveals higher hACE2 receptor binding affinity for Omicron B.1.1.529 spike RBD compared to wild type reference. Scientific reports, 12(1), 14534. [CrossRef]
- Dutta, D. Dutta, D., Liu, J., & Xiong, H. (2022). NLRP3 inflammasome activation and SARS-CoV-2-mediated hyperinflammation, cytokine storm and neurological syndromes. International journal of physiology, pathophysiology and pharmacology, 14(3), 138–160.
- Edahiro, Y. , Ohishi, K., Gotoh, A., Takenaka, K., Shibayama, H., Shimizu, T., Usuki, K., Shimoda, K., Ito, M., VanWart, S. A., Zagrijtschuk, O., Qin, A., Kawase, H., Miyachi, N., Sato, T., Komatsu, N., & Kirito, K. (2022). Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. International journal of hematology, 116(2), 215–227. [CrossRef]
- Edahiro, Y., Yasuda, H., Gotoh, A., Morishita, S., Suzuki, T., Takeda, J., Ando, J., Tsutsui, M., Itakura, A., & Komatsu, N. (2021). Interferon therapy for pregnant patients with essential thrombocythemia in Japan. International journal of hematology, 113(1), 106–111. [CrossRef]
- Effat Davoudi-Monfared, Hamid Rahmani, Hossein Khalili, Mahboubeh Hajiabdolbaghi, Mohamadreza Salehi, Ladan Abbasian, Hossein Kazemzadeh, Mir Saeed Yekaninejad (2020), A Randomized Clinical Trial of the Efficacy and Safety of Interferon Beta-1a in Treatment of Severe COVID-19, ASM Journals, Vol 64, 9. [CrossRef]
- Ehrhardt, C., Wolff, T., Pleschka, S., Planz, O., Beermann, W., Bode, J. G., Schmolke, M., & Ludwig, S. (2007). Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. Journal of virology, 81(7), 3058–3067. [CrossRef]
- Ejlerskov, P., Hultberg, J. G., Wang, J., Carlsson, R., Ambjørn, M., Kuss, M., Liu, Y., Porcu, G., Kolkova, K., Friis Rundsten, C., Ruscher, K., Pakkenberg, B., Goldmann, T., Loreth, D., Prinz, M., Rubinsztein, D. C., & Issazadeh-Navikas, S. (2015). Lack of Neuronal IFN-β-IFNAR Causes Lewy Body- and Parkinson's Disease-like Dementia. Cell, 163(2), 324–339. [CrossRef]
- Elkon, K. B., & Santer, D. M. (2012). Complement, interferon and lupus. Current opinion in immunology, 24(6), 665–670. [CrossRef]
- Emiliani, Yuliana and Muzi, Gabriel and Sanchez, Andres and Munera, Marlon, Prediction of Molecular Mimicry between Proteins from Trypanosoma Sp. And Human Antigens Associated with Systemic Lupus Erythematosus. Available at SSRN: https://ssrn.com/abstract=4184590 or. [CrossRef]
- Emilie, D., Burgard, M., Lascoux-Combe, C., Laughlin, M., Krzysiek, R., Pignon, C., Rudent, A., Molina, J. M., Livrozet, J. M., Souala, F., Chene, G., Grangeot-Keros, L., Galanaud, P., Sereni, D., Rouzioux, C., & Primoferon A Study Group (2001). Early control of HIV replication in primary HIV-1 infection treated with antiretroviral drugs and pegylated IFN alpha: results from the Primoferon A (ANRS 086) Study. AIDS (London, England), 15(11), 1435–1437. [CrossRef]
- Emily Mantlo, Natalya Bukreyeva, Junki Maruyama, et al. (2020), Antiviral Activities of Type I interferons to SARS-CoV-2 Infection, Journal of Antiviral Resistance, Vol. 178, 104811, doi:. [CrossRef]
- Ezelle, H. J., Balachandran, S., Sicheri, F., Polyak, S. J., & Barber, G. N. (2001). Analyzing the mechanisms of interferon-induced apoptosis using CrmA and hepatitis C virus NS5A. Virology, 281(1), 124–137. [CrossRef]
- Faleiro RJ, Kumar R, Bunn PT, Singh N, Chauhan SB, et al. (2016) Combined Immune Therapy for the Treatment of Visceral Leishmaniasis. PLOS Neglected Tropical Diseases 10(2): e0004415. [CrossRef]
- Fanunza, E., Frau, A., Corona, A., & Tramontano, E. (2019). Insights into Ebola Virus VP35 and VP24 Interferon Inhibitory Functions and their Initial Exploitation as Drug Targets. Infectious disorders drug targets, 19(4), 362–374. [CrossRef]
- Farinholt, T. , Doddapaneni, H., Qin, X., Menon, V., Meng, Q., Metcalf, G., Chao, H., Gingras, M.C., Avadhanula, V., Farinholt, P., Agrawal, C., Muzny, D.M., Piedra, P.A., Gibbs, R.A., & Petrosino, J. (2021) Transmission event of SARS-CoV-2 delta variant reveals multiple vaccine breakthrough infections. BMC Med, 19(1): 255. [CrossRef]
- Farooq, M. , Khan, A. W., Ahmad, B., Kim, M. S., & Choi, S. (2022). Therapeutic Targeting of Innate Immune Receptors Against SARS-CoV-2 Infection. Frontiers in pharmacology, 13, 915565. [CrossRef]
- Fatemi Naeini, F. , Yazdanpanah, M., Mohaghegh, F., Rajabi, P., & Tabatabaei, E. T. (2022). Interferon α-induced lupus-like reaction in a mycosis fungoides patient: A case report. International journal of clinical pharmacology and therapeutics, 60(7), 306–310. [CrossRef]
- Fazeli, M. R., & Hezarjaribi, N. (2022). A Simplified Process for Purification and Refolding of Recombinant Human Interferon-α2b. Iranian biomedical journal, 26(1), 85–90. [CrossRef]
- Fehniger, T. A., Herbein, G., Yu, H., Para, M. I., Bernstein, Z. P., O'Brien, W. A., & Caligiuri, M. A. (1998). Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. Journal of immunology (Baltimore, Md. : 1950), 161(11), 6433–6438.
- Felgenhauer, U., Schoen, A., Gad, H. H., Hartmann, R., Schaubmar, A. R., Failing, K., Drosten, C., & Weber, F. (2020). Inhibition of SARS-CoV-2 by type I and type III interferons. The Journal of biological chemistry, 295(41), 13958–13964. [CrossRef]
- Felgenhauer, U. , Schoen, A., Gad, H. H., Hartmann, R., Schaubmar, A. R., Failing, K., Drosten, C., & Weber, F. (2020). Inhibition of SARS-CoV-2 by type I and type III interferons. The Journal of biological chemistry, 295(41), 13958–13964. [CrossRef]
- Felgenhauer, U., Schoen, A., Gad, H. H., Hartmann, R., Schaubmar, A. R., Failing, K., Drosten, C., & Weber, F. (2020). Inhibition of SARS-CoV-2 by type I and type III interferons. The Journal of biological chemistry, 295(41), 13958–13964. [CrossRef]
- Feng, E., Balint, E., Poznanski, S. M., Ashkar, A. A., & Loeb, M. (2021). Aging and Interferons: Impacts on Inflammation and Viral Disease Outcomes. Cells, 10(3), 708. [CrossRef]
- Fenton-May, A. E., Dibben, O., Emmerich, T., Ding, H., Pfafferott, K., Aasa-Chapman, M. M., Pellegrino, P., Williams, I., Cohen, M. S., Gao, F., Shaw, G. M., Hahn, B. H., Ochsenbauer, C., Kappes, J. C., & Borrow, P. (2013). Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology, 10, 146. [CrossRef]
- Ferran, M. C., & Lucas-Lenard, J. M. (1997). The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. Journal of virology, 71(1), 371–377. [CrossRef]
- Fitzgerald-Bocarsly, P. , & Feng, D. (2007). The role of type I interferon production by dendritic cells in host defense. Biochimie, 89(6-7), 843–855. [CrossRef]
- Flachenecker P. (2004). Disease-modifying drugs for the early treatment of multiple sclerosis. Expert review of neurotherapeutics, 4(3), 455–463. [CrossRef]
- Fleming, D; A Scientific and Forensic investigation: R.M. (2021). Is COVID-19 a Bioweapon? ISBN 978-1-5107-7019-5.
- Flemming A. (2022). Omicron, the great escape artist. Nature reviews. Immunology, 22(2), 75. [CrossRef]
- Francis, M. L., Meltzer, M. S., & Gendelman, H. E. (1992). Interferons in the persistence, pathogenesis, and treatment of HIV infection. AIDS research and human retroviruses, 8(2), 199–207. [CrossRef]
- Frieman, M., Yount, B., Heise, M., Kopecky-Bromberg, S. A., Palese, P., & Baric, R. S. (2007). Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. Journal of virology, 81(18), 9812–9824. [CrossRef]
- Frisch, S. M., & MacFawn, I. P. (2020). Type I interferons and related pathways in cell senescence. Aging cell, 19(10), e13234. [CrossRef]
- Frumholtz, L. , Bouaziz, J. D., Battistella, M., Hadjadj, J., Chocron, R., Bengoufa, D., Le Buanec, H., Barnabei, L., Meynier, S., Schwartz, O., Grzelak, L., Smith, N., Charbit, B., Duffy, D., Yatim, N., Calugareanu, A., Philippe, A., Guerin, C. L., Joly, B., Siguret, V., … Saint-Louis CORE (COvid REsearch) (2021). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. The British journal of dermatology, 185(6), 1176–1185. [CrossRef]
- Fukuda, Y., Homma, T., Inoue, H., Goto, Y., Sato, Y., Ikeda, H., Onitsuka, C., Sato, H., Akimoto, K., Ebato, T., Suganuma, H., Kawahara, T., Mikuni, H., Uchida, Y., Suzuki, S., Tanaka, A., & Sagara, H. (2022). Serum IL-28A/IFN-λ2 is linked to disease severity of COVID-19. Scientific reports, 12(1), 5458. [CrossRef]
- Fukuda, Y., Homma, T., Inoue, H., Onitsuka, C., Ikeda, H., Goto, Y., Sato, Y., Kimura, T., Hirai, K., Ohta, S., Yamamoto, M., Kusumoto, S., Suzuki, S., Tanaka, A., & Sagara, H. (2021). Downregulation of type III interferons in patients with severe COVID-19. Journal of medical virology, 93(7), 4559–4563. [CrossRef]
- Fukuda, Y. , Homma, T., Inoue, H., Onitsuka, C., Ikeda, H., Goto, Y., Sato, Y., Kimura, T., Hirai, K., Ohta, S., Yamamoto, M., Kusumoto, S., Suzuki, S., Tanaka, A., & Sagara, H. (2021). Downregulation of type III interferons in patients with severe COVID-19. Journal of medical virology, 93(7), 4559–4563. [CrossRef]
- Galão, R. P., Wilson, H., Schierhorn, K. L., Debeljak, F., Bodmer, B. S., Goldhill, D., Hoenen, T., Wilson, S. J., Swanson, C. M., & Neil, S. (2022). TRIM25 and ZAP target the Ebola virus ribonucleoprotein complex to mediate interferon-induced restriction. PLoS pathogens, 18(5), e1010530. [CrossRef]
- Gal-Ben-Ari, S., Barrera, I., Ehrlich, M., & Rosenblum, K. (2019). PKR: a kinase to remember. Frontiers in molecular neuroscience, 11, 480.
- Gamdzyk, M., Doycheva, D. M., Araujo, C., Ocak, U., Luo, Y., Tang, J., & Zhang, J. H. (2020). cGAS/STING Pathway Activation Contributes to Delayed Neurodegeneration in Neonatal Hypoxia-Ischemia Rat Model: Possible Involvement of LINE-1. Molecular neurobiology, 57(6), 2600–2619. [CrossRef]
- Gao, L., Yu, S., Chen, Q., Duan, Z., Zhou, J., Mao, C., Yu, D., Zhu, W., Nie, J., & Hou, Y. (2010). A randomized controlled trial of low-dose recombinant human interferons alpha-2b nasal spray to prevent acute viral respiratory infections in military recruits. Vaccine, 28(28), 4445–4451. [CrossRef]
- Gardinassi, L. G., Souza, C., Sales-Campos, H., & Fonseca, S. G. (2020). Immune and Metabolic Signatures of COVID-19 Revealed by Transcriptomics Data Reuse. Frontiers in immunology, 11, 1636. [CrossRef]
- Garg, A. D., & Agostinis, P. (2017). Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunological reviews, 280(1), 126–148. [CrossRef]
- Gargan, S., Ahmed, S., Mahony, R., Bannan, C., Napoletano, S., O'Farrelly, C., Borrow, P., Bergin, C., & Stevenson, N. J. (2018). HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-viral ISG Induction. EBioMedicine, 30, 203–216. [CrossRef]
- Gargan, S., Ahmed, S., Mahony, R., Bannan, C., Napoletano, S., O'Farrelly, C., Borrow, P., Bergin, C., & Stevenson, N. J. (2018). HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-viral ISG Induction. EBioMedicine, 30, 203–216. [CrossRef]
- Gargano, J. W., Wallace, M., Hadler, S.C., Langley, G., Su, J.R., Oster, M.E., Broder, K.R., Gee, J., Weintraub, E., Shimabukuro, T., Scobie, H.M., Moulia, D., Markowitz, L.E., Wharton, M., McNally, V.V., Romero, J.R., Talbot, H.K., Lee, G.M., Daley, M.F., & Oliver, S.E. (2021). Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, MMWR Morb Mortal Wkly Rep, 70(27): 977-982. [CrossRef]
- Gartlan, C., Tipton, T., Salguero, F. J., Sattentau, Q., Gorringe, A., & Carroll, M. W. (2022). Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Frontiers in immunology, 13, 882972. [CrossRef]
- Gaudieri, S., Lucas, M., Lucas, A., McKinnon, E., Albloushi, H., Rauch, A., di Iulio, J., Martino, D., Prescott, S. L., & Tulic, M. K. (2012). Genetic variations in IL28B and allergic disease in children. PloS one, 7(1), e30607. [CrossRef]
- George J and Mattapallil JJ (2018), Interferon-α Subtypes As an Adjunct Therapeutic Approach for Human Immunodeficiency Virus Functional Cure. Front. Immunol. 9, 299. [CrossRef] [PubMed]
- Ghasemitarei, M., Privat-Maldonado, A., Yusupov, M., Rahnama, S., Bogaerts, A., & Ejtehadi, M. R. (2022). Effect of Cysteine Oxidation in SARS-CoV-2 Receptor-Binding Domain on Its Interaction with Two Cell Receptors: Insights from Atomistic Simulations. Journal of chemical information and modeling, 62(1), 129–141. [CrossRef]
- Gigante, M. , Mandic, M., Wesa, A. K., Cavalcanti, E., Dambrosio, M., Mancini, V., Battaglia, M., Gesualdo, L., Storkus, W. J., & Ranieri, E. (2008). Interferon-alpha (IFN-alpha)-conditioned DC preferentially stimulate type-1 and limit Treg-type in vitro T-cell responses from RCC patients. Journal of immunotherapy (Hagerstown, Md. : 1997), 31(3), 254–262. [CrossRef]
- Gil, J., & Esteban, M. (2000). The interferon-induced protein kinase (PKR), triggers apoptosis through FADD-mediated activation of caspase 8 in a manner independent of Fas and TNF-alpha receptors. Oncogene, 19(32), 3665–3674. [CrossRef]
- Gilbert, C., Lefeuvre, C., Preisser, L., Pivert, A., Soleti, R., Blanchard, S., Delneste, Y., Ducancelle, A., Couez, D., & Jeannin, P. (2021). Age-Related Expression of IFN-λ1 Versus IFN-I and Beta-Defensins in the Nasopharynx of SARS-CoV-2-Infected Individuals. Frontiers in immunology, 12, 750279. [CrossRef]
- Gilbert, C., Lefeuvre, C., Preisser, L., Pivert, A., Soleti, R., Blanchard, S., Delneste, Y., Ducancelle, A., Couez, D., & Jeannin, P. (2021). Age-Related Expression of IFN-λ1 Versus IFN-I and Beta-Defensins in the Nasopharynx of SARS-CoV-2-Infected Individuals. Frontiers in immunology, 12, 750279. [CrossRef]
- Gill, N., Deacon, P. M., Lichty, B., Mossman, K. L., & Ashkar, A. A. (2006). Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. Journal of virology, 80(20), 9943–9950. [CrossRef]
- Gina Kolata, “Ignored AIDS Drug Shows Promise in Small Tests,” New York Times, August 15, 1989; //www: https.
- Giotis, E. S., Robey, R. C., Skinner, N. G., Tomlinson, C. D., Goodbourn, S., & Skinner, M. A. (2016). Chicken interferome: avian interferon-stimulated genes identified by microarray and RNA-seq of primary chick embryo fibroblasts treated with a chicken type I interferon (IFN-α). Veterinary research, 47(1), 75. [CrossRef]
- Glennon, N. B., Jabado, O., Lo, M. K., & Shaw, M. L. (2015). Transcriptome Profiling of the Virus-Induced Innate Immune Response in Pteropus vampyrus and Its Attenuation by Nipah Virus Interferon Antagonist Functions. Journal of virology, 89(15), 7550–7566. [CrossRef]
- Glennon-Alty, L., Moots, R. J., Edwards, S. W., & Wright, H. L. (2021). Type I interferon regulates cytokine-delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression. Clinical and experimental immunology, 203(2), 151–159. [CrossRef]
- Goh, J., & Behringer, M. (2018). Exercise alarms the immune system: A HMGB1 perspective. Cytokine, 110, 222–225. [CrossRef]
- Goldman, S. , Bron, D., Tousseyn, T., Vierasu, I., Dewispelaere, L., Heimann, P., Cogan, E., & Goldman, M. (2021). Rapid progression of angioimmunoblastic T Cell lymphoma following BNT162b2 mRNA vaccine booster shot: a case report. Frontiers in Medicine, 8, 798095. [CrossRef]
- Gorlé, N., & Vandenbroucke, R. E. (2019). Interferons: A molecular switch between damage and repair in ageing and Alzheimer's disease. Mechanisms of ageing and development, 183, 111148. [CrossRef]
- Govek, E. E., Hatten, M. E., & Van Aelst, L. (2011). The role of Rho GTPase proteins in CNS neuronal migration. Developmental neurobiology, 71(6), 528–553. [CrossRef]
- Grant, A. H., Estrada, A., 3rd, Ayala-Marin, Y. M., Alvidrez-Camacho, A. Y., Rodriguez, G., Robles-Escajeda, E., Cadena-Medina, D. A., Rodriguez, A. C., & Kirken, R. A. (2021). The Many Faces of JAKs and STATs Within the COVID-19 Storm. Frontiers in immunology, 12, 690477. [CrossRef]
- Grant, A., Ponia, S. S., Tripathi, S., Balasubramaniam, V., Miorin, L., Sourisseau, M., Schwarz, M. C., Sánchez-Seco, M. P., Evans, M. J., Best, S. M., & García-Sastre, A. (2016). Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell host & microbe, 19(6), 882–890. [CrossRef]
- Grenfell, B. T., Pybus, O.G., Gog, J.R., Wood, J.L., Daly, J.M., Mumford, J.A., & Holmes, E.C. (2004). Unifying the epidemiological and evolutionary dynamics of pathogens. Science, 303(5656): 327-332. [CrossRef]
- Grifoni, A. , Weiskopf, D., Ramirez, S.I., Mateus, J., Dan, J.M., Moderbacher, C.R., Rawlings, S.A., Sutherland, A., Premkumar, L., Jadi, R.S., Marrama, D., de Silva, A.M., Frazier, A., Carlin, A.F., Greenbaum, J.A., Peters, B., Krammer, F., Smith, D.M., Crotty, S., & Sette, A. (2020). Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell, 181(7):1489-1501.e15. [CrossRef]
- Guo, Y. , Yang, C., Liu, Y., Li, T., Li, H., Han, J., Jia, L., Wang, X., Zhang, B., Li, J., & Li, L. (2022). High Expression of HERV-K (HML-2) Might Stimulate Interferon in COVID-19 Patients. Viruses, 14(5), 996. [CrossRef]
- Gupta, S., Nakabo, S., Chu, J., Hasni, S., & Kaplan, M. J. (2020). Association between anti-interferon-alpha autoantibodies and COVID-19 in systemic lupus erythematosus. medRxiv : the preprint server for health sciences, 2020.10.29.20222000. [CrossRef]
- Haasbach, E., Droebner, K., Vogel, A. B., & Planz, O. (2011). Low-dose interferon Type I treatment is effective against H5N1 and swine-origin H1N1 influenza A viruses in vitro and in vivo. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 31(6), 515–525. [CrossRef]
- Habjan, M., Andersson, I., Klingström, J., Schümann, M., Martin, A., Zimmermann, P., Wagner, V., Pichlmair, A., Schneider, U., Mühlberger, E., Mirazimi, A., & Weber, F. (2008). Processing of genome 5' termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PloS one, 3(4), e2032. [CrossRef]
- Hackstadt, T., Chiramel, A. I., Hoyt, F. H., Williamson, B. N., Dooley, C. A., Beare, P. A., de Wit, E., Best, S. M., & Fischer, E. R. (2021). Disruption of the Golgi Apparatus and Contribution of the Endoplasmic Reticulum to the SARS-CoV-2 Replication Complex. Viruses, 13(9), 1798. [CrossRef]
- Haga, R. B., & Ridley, A. J. (2016). Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases, 7(4), 207–221. [CrossRef]
- Hakami A. R. (2022). Targeting the RBD of Omicron Variant (B.1.1.529) with Medicinal Phytocompounds to Abrogate the Binding of Spike Glycoprotein with the hACE2 Using Computational Molecular Search and Simulation Approach. Biology, 11(2), 258. [CrossRef]
- Haller O. (2015). A tribute to Jean Lindenmann, co-discoverer of interferon (1924-2015). Cytokine, 76(1), 113–115. [CrossRef]
- Haller, O., & Kochs, G. (2011). Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 31(1), 79–87. [CrossRef]
- Haller, O., Gao, S., von der Malsburg, A., Daumke, O., & Kochs, G. (2010). Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity. The Journal of biological chemistry, 285(37), 28419–28424. [CrossRef]
- Halstead S. B. (2021). Vaccine-Associated Enhanced Viral Disease: Implications for Viral Vaccine Development. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy, 35(5), 505–515. [CrossRef]
- Han, L. , Zheng, Y., Deng, J., Nan, M. L., Xiao, Y., Zhuang, M. W., Zhang, J., Wang, W., Gao, C., & Wang, P. H. (2022). SARS-CoV-2 ORF10 antagonizes STING-dependent interferon activation and autophagy. Journal of medical virology, 10.1002/jmv.27965. Advance online publication. [CrossRef]
- Han, L. , Zhuang, M. W., Deng, J., Zheng, Y., Zhang, J., Nan, M. L., Zhang, X. J., Gao, C., & Wang, P. H. (2021). SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. Journal of medical virology, 93(9), 5376–5389. [CrossRef]
- Harandi A. M. (2004). The potential of immunostimulatory CpG DNA for inducing immunity against genital herpes: opportunities and challenges. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 30(3), 207–210. [CrossRef]
- Harman, A. N., Nasr, N., Feetham, A., Galoyan, A., Alshehri, A. A., Rambukwelle, D., Botting, R. A., Hiener, B. M., Diefenbach, E., Diefenbach, R. J., Kim, M., Mansell, A., & Cunningham, A. L. (2015). HIV Blocks Interferon Induction in Human Dendritic Cells and Macrophages by Dysregulation of TBK1. Journal of virology, 89(13), 6575–6584. [CrossRef]
- Hayden, F. G., & Gwaltney, J. M., Jr (1984). Intranasal interferon-alpha 2 treatment of experimental rhinoviral colds. The Journal of infectious diseases, 150(2), 174–180. [CrossRef]
- Hayden, F. G., Kaiser, D. L., & Albrecht, J. K. (1988). Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicrobial agents and chemotherapy, 32(2), 224–230. [CrossRef]
- He, B. , Tran, J. T., & Sanchez, D. J. (2019). Manipulation of Type I Interferon Signaling by HIV and AIDS-Associated Viruses. Journal of immunology research, 2019, 8685312. [CrossRef]
- He, Z. , Tian, M., & Fu, X. (2021). Reduced expression of miR-30c-5p promotes hepatocellular carcinoma progression by targeting RAB32. Molecular therapy. Nucleic acids, 26, 603–612. [CrossRef]
- Hefti, H. P., Frese, M., Landis, H., Di Paolo, C., Aguzzi, A., Haller, O., & Pavlovic, J. (1999). Human MxA protein protects mice lacking a functional alpha/beta interferon system against La crosse virus and other lethal viral infections. Journal of virology, 73(8), 6984–6991. [CrossRef]
- Herder, V. , Dee, K., Wojtus, J. K., Epifano, I., Goldfarb, D., Rozario, C., Gu, Q., Da Silva Filipe, A., Nomikou, K., Nichols, J., Jarrett, R. F., Stevenson, A., McFarlane, S., Stewart, M. E., Szemiel, A. M., Pinto, R. M., Masdefiol Garriga, A., Davis, C., Allan, J., Graham, S. V., … Boutell, C. (2021). Elevated temperature inhibits SARS-CoV-2 replication in respiratory epithelium independently of IFN-mediated innate immune defenses. PLoS biology, 19(12), e3001065. [CrossRef]
- Higgins, P. G., Al-Nakib, W., Willman, J., & Tyrrell, D. A. (1986). Interferon-beta ser as prophylaxis against experimental rhinovirus infection in volunteers. Journal of interferon research, 6(2), 153–159. [CrossRef]
- Hijano, D. R., Vu, L. D., Kauvar, L. M., Tripp, R. A., Polack, F. P., & Cormier, S. A. (2019). Role of Type I Interferon (IFN) in the Respiratory Syncytial Virus (RSV) Immune Response and Disease Severity. Frontiers in immunology, 10, 566. [CrossRef]
- Hirose, S. , Hara, M., Koda, K., Natori, N., Yokota, Y., Ninomiya, S., & Nakajima, H. (2021). Acute autoimmune transverse myelitis following COVID-19 vaccination: A case report. Medicine (Baltimore). 100(51): e28423. [CrossRef]
- Ho, M.-W. Ho, M.-W. (1999). Genetic Engineering, Dream or Nightmare? The Brave New World of Bad Science and Big Business, 2nd ed. Gateway Books, Dublin (IRL). 8264. [Google Scholar]
- Ho, M. -W. (2013). The new genetics and natural versus artificial genetic modification. Entropy, 15(11), 4748–4781. [CrossRef]
- Hoffman, R. M., & Han, Q. (2020). Oral Methioninase for Covid-19 Methionine-restriction Therapy. In vivo (Athens, Greece), 34(3 Suppl), 1593–1596. [CrossRef]
- Hoffman, R. M., & Han, Q. (2020). Oral Methioninase for Covid-19 Methionine-restriction Therapy. In vivo (Athens, Greece), 34(3 Suppl), 1593–1596. [CrossRef]
- Hossain, A., Akter, S., Rashid, A. A., Khair, S., & Alam, A. (2022). Unique mutations in SARS-CoV-2 Omicron subvariants' non-spike proteins: Potential impacts on viral pathogenesis and host immune evasion. Microbial pathogenesis, 170, 105699. [CrossRef]
- Hotter, D., Bosso, M., Jønsson, K. L., Krapp, C., Stürzel, C. M., Das, A., Littwitz-Salomon, E., Berkhout, B., Russ, A., Wittmann, S., Gramberg, T., Zheng, Y., Martins, L. J., Planelles, V., Jakobsen, M. R., Hahn, B. H., Dittmer, U., Sauter, D., & Kirchhoff, F. (2019). IFI16 Targets the Transcription Factor Sp1 to Suppress HIV-1 Transcription and Latency Reactivation. Cell host & microbe, 25(6), 858–872.e13. [CrossRef]
- Hou, W., Wang, S., Wu, H., Xue, L., Wang, B., Wang, S., & Wang, H. (2022). Small GTPase-A Key Role in Host Cell for Coronavirus Infection and a Potential Target for Coronavirus Vaccine Adjuvant Discovery. Viruses, 14(9), 2044. [CrossRef]
- How, J., & Hobbs, G. (2020). Use of Interferon Alfa in the Treatment of Myeloproliferative Neoplasms: Perspectives and Review of the Literature. Cancers, 12(7), 1954. [CrossRef]
- Hu, W. S., & Hughes, S. H. (2012). HIV-1 reverse transcription. Cold Spring Harbor perspectives in medicine, 2(10), a006882. [CrossRef]
- Huang, J., You, H., Su, C., Li, Y., Chen, S., & Zheng, C. (2018). Herpes Simplex Virus 1 Tegument Protein VP22 Abrogates cGAS/STING-Mediated Antiviral Innate Immunity. Journal of virology, 92(15), e00841-18. [CrossRef]
- Huang, M. , Jiang, J. D., & Peng, Z. (2014). Recent advances in the anti-HCV mechanisms of interferon. Acta pharmaceutica Sinica. B, 4(4), 241–247. [CrossRef]
- Huang, Y., Xie, J., Guo, Y., Sun, W., He, Y., Liu, K., Yan, J., Tao, A., & Zhong, N. (2021). SARS-CoV-2: Origin, Intermediate Host and Allergenicity Features and Hypotheses. Healthcare (Basel, Switzerland), 9(9), 1132. [CrossRef]
- Hui, K., Cheung, M. C., Perera, R., Ng, K. C., Bui, C., Ho, J., Ng, M., Kuok, D., Shih, K. C., Tsao, S. W., Poon, L., Peiris, M., Nicholls, J. M., & Chan, M. (2020). Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. The Lancet. Respiratory medicine, 8(7), 687–695. [CrossRef]
- Huyton, T., Göttmann, W., Bade-Döding, C., Paine, A., & Blasczyk, R. (2011). The T/NK cell co-stimulatory molecule SECTM1 is an IFN “early response gene” that is negatively regulated by LPS in human monocytic cells. Biochimica Et Biophysica Acta (BBA) - General Subjects, 1810(12), 1294–1301. [CrossRef]
- Iaconis, G., Jackson, B., Childs, K., Boyce, M., Goodbourn, S., Blake, N., Iturriza-Gomara, M., & Seago, J. (2021). Rotavirus NSP1 Inhibits Type I and Type III Interferon Induction. Viruses, 13(4), 589. [CrossRef]
- Ilaria Sciamanna, Chiara de Luca and Corrado Spadafora (2016), The Reverse Transcriptase Encodes by LINE-1 Retrotransposons in the Genesis, Progression and Therapy of Cancer, Front. Chem., doi:. [CrossRef]
- Ilinykh, P. A., Lubaki, N. M., Widen, S. G., Renn, L. A., Theisen, T. C., Rabin, R. L., Wood, T. G., & Bukreyev, A. (2015). Different Temporal Effects of Ebola Virus VP35 and VP24 Proteins on Global Gene Expression in Human Dendritic Cells. Journal of virology, 89(15), 7567–7583. [CrossRef]
- Illés, Á., Pinczés, L. I., & Egyed, M. (2021). A pharmacokinetic evaluation of ropeginterferon alfa-2b in the treatment of polycythemia vera. Expert opinion on drug metabolism & toxicology, 17(1), 3–7. [CrossRef]
- Inserra, F. , Tajer, C., Antonietti, L., Mariani, J., Ferder, L., & Manucha, W. (2021). Vitamin D supplementation: An alternative to enhance the effectiveness of vaccines against SARS-CoV-2? Vaccine, 39(35): 4930-4931. [CrossRef]
- Islam, A. , Khan, M. A., Ahmed, R., Hossain, M. S., Kabir, S., Islam, M. S., & Siddiki, A. (2021). Transcriptome of nasopharyngeal samples from COVID-19 patients and a comparative analysis with other SARS-CoV-2 infection models reveal disparate host responses against SARS-CoV-2. Journal of translational medicine, 19(1), 32. [CrossRef]
- Ito, H., Morishita, R., Tabata, H., & Nagata, K. (2014). Roles of Rho small GTPases in the tangentially migrating neurons. Histology and histopathology, 29(7), 871–879. [CrossRef]
- Ivashkiv, L. B., & Donlin, L. T. (2014). Regulation of type I interferon responses. Nature reviews. Immunology, 14(1), 36–49. [CrossRef]
- Iverson, E., Griswold, K., Song, D., Gagliardi, T. B., Hamidzadeh, K., Kesimer, M., Sinha, S., Perry, M., Duncan, G. A., & Scull, M. A. (2022). Membrane-Tethered Mucin 1 Is Stimulated by Interferon and Virus Infection in Multiple Cell Types and Inhibits Influenza A Virus Infection in Human Airway Epithelium. mBio, 13(4), e0105522. [CrossRef]
- Ives Charlie-Silva, Amanda P. C. Araújo, Abraão T. B. Guimarães, Flávio P Veras, Helyson L. B. Braz, Letícia G. de Pontes, Roberta J. B. Jorge, Marco A. A. Belo, Bianca H, V. Fernandes, Rafael H. Nóbrega, Giovane Galdino, Antônio Condino-Neto, Jorge Galindo-Villegas, Glaucia M. Machado-Santelli, Paulo R. S. Sanches, Rafael M. Rezende, Eduardo M. Cilli, Guilherme Malafaia, An insight into neurotoxic and toxicity of spike fragments SARS-CoV-2 by exposure environment: A threat to aquatic health?, available at: bioRxiv 2021.01.11.425914; doi:. [CrossRef]
- Jafarzadeh, A., Chauhan, P., Saha, B., Jafarzadeh, S., & Nemati, M. (2020). Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life sciences, 257, 118102. [CrossRef]
- Jafarzadeh, A. , Nemati, M., Saha, B., Bansode, Y. D., & Jafarzadeh, S. (2021). Protective Potentials of Type III Interferons in COVID-19 Patients: Lessons from Differential Properties of Type I- and III Interferons. Viral immunology, 34(5), 307–320. [CrossRef]
- Jafarzadeh, A. , Nemati, M., Saha, B., Bansode, Y. D., & Jafarzadeh, S. (2021). Protective Potentials of Type III Interferons in COVID-19 Patients: Lessons from Differential Properties of Type I- and III Interferons. Viral immunology, 34(5), 307–320. [CrossRef]
- Jiang, S. (2020). Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature, 579, 321. [CrossRef]
- Jiao, P., Tian, G., Li, Y., Deng, G., Jiang, Y., Liu, C., Liu, W., Bu, Z., Kawaoka, Y., & Chen, H. (2008). A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. Journal of virology, 82(3), 1146–1154. [CrossRef]
- The Journal of Infectious Diseases, 128; //www: (2), 261–264. http.
- Jureidini J, McHenry L B. The illusion of evidence based medicine BMJ 2022; 376 :o702. [CrossRef]
- Kalashnyk, O., Lykhmus, O., Izmailov, M., Koval, L., Komisarenko, S., & Skok, M. (2021). SARS-Cov-2 spike protein fragment 674-685 protects mitochondria from releasing cytochrome c in response to apoptogenic influence. Biochemical and biophysical research communications, 561, 14–18. [CrossRef]
- Kalyuzhin, O. V., Ponezheva, L. O., Turapova, A. N., Nurtazina, A. Y., Bykov, A. S., & Karaulov, A. V. (2022). Interferons alpha and Gamma, pidotimod, and tilorone in the treatment of acute respiratory infections in patients with allergic rhinitis: A prospective, cohort clinical and immunological study. Bulletin of Siberian Medicine, 21(2), 48–59. [CrossRef]
- Karlowitz, R. , Stanifer, M. L., Roedig, J., Andrieux, G., Bojkova, D., Bechtel, M., Smith, S., Kowald, L., Schubert, R., Boerries, M., Cinatl, J., Jr, Boulant, S., & van Wijk, S. (2022). USP22 controls type III interferon signaling and SARS-CoV-2 infection through activation of STING. Cell death & disease, 13(8), 684. [CrossRef]
- Katlinski, K. V., Gui, J., Katlinskaya, Y. V., Ortiz, A., Chakraborty, R., Bhattacharya, S., Carbone, C. J., Beiting, D. P., Girondo, M. A., Peck, A. R., Puré, E., Chatterji, P., Rustgi, A. K., Diehl, J. A., Koumenis, C., Rui, H., & Fuchs, S. Y. (2017). Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer cell, 31(2), 194–207. [CrossRef]
- Kato, A. , Ohnishi, Y., Kohase, M., Saito, S., Tashiro, M., & Nagai, Y. (2001). Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis. Journal of virology, 75(8), 3802–3810. [CrossRef]
- 2021; -9. [CrossRef]
- Khaledi, M., Sameni, F., Yahyazade, S., Radandish, M., Owlia, P., Bagheri, N., Afkhami, H., Mahjoor, M., Esmaelpour, Z., Kohansal, M., & Aghaei, F. (2022). COVID-19 and the potential of Janus family kinase (JAK) pathway inhibition: A novel treatment strategy. Frontiers in medicine, 9, 961027. [CrossRef]
- Khalil, B. A., Elemam, N. M., & Maghazachi, A. A. (2021). Chemokines and chemokine receptors during COVID-19 infection. Computational and structural biotechnology journal, 19, 976–988. [CrossRef]
- Khanmohammadi, S., Rezaei, N., Khazaei, M. et al. A Case of Autosomal Recessive Interferon Alpha/Beta Receptor Alpha Chain (IFNAR1) Deficiency with Severe COVID-19. J Clin Immunol 42, 19–24 (2022). [CrossRef]
- Khatamzas, E. , Hipp, M. M., Gaughan, D., Pichulik, T., Leslie, A., Fernandes, R. A., Muraro, D., Booth, S., Zausmer, K., Sun, M. Y., Kessler, B., Rowland-Jones, S., Cerundolo, V., & Simmons, A. (2017). Snapin promotes HIV-1 transmission from dendritic cells by dampening TLR8 signaling. The EMBO journal, 36(20), 2998–3011. [CrossRef]
- Kho, V. M., Mekers, V. E., Span, P. N., Bussink, J., & Adema, G. J. (2021). Radiotherapy and cGAS/STING signaling: Impact on MDSCs in the tumor microenvironment. Cellular immunology, 362, 104298. [CrossRef]
- Kieseier, B. C., & Hartung, H. P. (2003). Current disease-modifying therapies in multiple sclerosis. Seminars in neurology, 23(2), 133–146. [CrossRef]
- Kim, E. S., Jeon, M. T., Kim, K. S., Lee, S., Kim, S., & Kim, D. G. (2021). Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses, 13(10), 2021. [CrossRef]
- Kim, H., Jang, J., Song, M. J., Kim, G., Park, C. H., Lee, D. H., Lee, S. H., & Chung, J. H. (2022). Attenuation of intrinsic ageing of the skin via elimination of senescent dermal fibroblasts with senolytic drugs. Journal of the European Academy of Dermatology and Venereology : JEADV, 36(7), 1125–1135. [CrossRef]
- Kim, H., Jang, J., Song, M. J., Park, C. H., Lee, D. H., Lee, S. H., & Chung, J. H. (2022). Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 150, 113034. [CrossRef]
- Kim, J. C., Park, T. J., & Kang, H. Y. (2022). Skin-Aging Pigmentation: Who Is the Real Enemy? Cells, 11(16), 2541. [CrossRef]
- Kim, J. H., Kim, D. H., Baek, S. H., Lee, H. J., Kim, M. R., Kwon, H. J., & Lee, C. H. (2006). Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-kappaB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochemical pharmacology, 71(8), 1198–1205. [CrossRef]
- Kim, J. Y., Kwon, J. S., Bae, S., Cha, H. H., Lim, J. S., Kim, M. C., Chung, J. W., Park, S. Y., Lee, M. J., Kim, B. N., Jung, J., Kim, M. J., Shin, E. C., & Kim, S. H. (2021). SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity. The American journal of tropical medicine and hygiene, 105(2), 395–400. [CrossRef]
- Kim, M. A., Lee, Y.W., Kim, S.R., Kim, J.H., Min, T.K., Park, H.S., Shin, M., Ye, Y.M., Lee, S., Lee, J., Choi, J.H., Jang, G.C., & Chang, Y.S. (2021). COVID-19 vaccine-associated anaphylaxis and allergic reactions: consensus statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol Res, 13(4):526-544. [CrossRef]
- Kim, Y. M., & Shin, E. C. (2021). Type I and III interferon responses in SARS-CoV-2 infection. Experimental & molecular medicine, 53(5), 750–760. [CrossRef]
- Kim, YM., Shin, EC. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med 53, 750–760 (2021). [CrossRef]
- Kirchdoerfer, R. N., Wang, N., Pallesen, J., Wrapp, D., Turner, H. L., Cottrell, C. A., Corbett, K. S., Graham, B. S., McLellan, J. S., & Ward, A. B. (2018). Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Scientific reports, 8(1), 15701. [CrossRef]
- Klinkhammer, J., Schnepf, D., Ye, L., Schwaderlapp, M., Gad, H. H., Hartmann, R., Garcin, D., Mahlakõiv, T., & Staeheli, P. (2018). IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife, 7, e33354. [CrossRef]
- Molecular biotherapy, 2; //pubmed: (2), 91–95, doi: https.
- Koga, R. , Ohno, S., Ikegame, S., & Yanagi, Y. (2010). Measles virus-induced immunosuppression in SLAM knock-in mice. Journal of virology, 84(10), 5360–5367. [CrossRef]
- Kong, Z., Yin, H., Wang, F., Liu, Z., Luan, X., Sun, L., Liu, W., & Shang, Y. (2022). Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS pathogens, 18(5), e1010544. [CrossRef]
- Korth, M. J., Taylor, M. D., & Katze, M. G. (1998). Interferon inhibits the replication of HIV-1, SIV, and SHIV chimeric viruses by distinct mechanisms. Virology, 247(2), 265–273. [CrossRef]
- Kosciuczuk, E. M., Mehrotra, S., Saleiro, D., Kroczynska, B., Majchrzak-Kita, B., Lisowski, P., Driehaus, C., Rogalska, A., Turner, A., Lienhoop, T., Gius, D., Fish, E. N., Vassilopoulos, A., & Platanias, L. C. (2019). Sirtuin 2-mediated deacetylation of cyclin-dependent kinase 9 promotes STAT1 signaling in type I interferon responses. The Journal of biological chemistry, 294(3), 827–837. [CrossRef]
- Kostoff, R. N., Calina, D., Kanduc, D., Briggs, M.B., Vlachoyiannopoulos, P., Svistunov, A.A., & Tsatsakis, A. (2021). Why are we vaccinating children against COVID-19? Toxicol Rep 8, 1665–1684. [CrossRef]
- Kotenko, S. V., Rivera, A., Parker, D., & Durbin, J. E. (2019). Type III IFNs: Beyond antiviral protection. Seminars in immunology, 43, 101303. [CrossRef]
- Kounis, N. G., Koniari, I., Mplani, V., Plotas, P., & Velissaris, D. (2022). Hypersensitivity myocarditis and the pathogenetic conundrum of COVID 19 Vaccine Related Myocarditis. Cardiology, 10.1159/000524224. Advance online publication. [CrossRef]
- Kouwaki, T. , Nishimura, T., Wang, G., & Oshiumi, H. (2021). RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Frontiers in immunology, 12, 700926. [CrossRef]
- Kowarz, E. , Krutzke, L., Reis, J., Bracharz, S., Kochanek, S., & Marschalek, R. (2021). Vaccine-induced COVID-19 mimicry syndrome: splice reactions within the SARS-CoV-2 spike open reading frame result in spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Nature-portfolio [preprint]. [CrossRef]
- Krutzke, L. , Roesler, R., Wiese, S., & Kochanek, S. (2021). Process-related impurities in the ChAdOx1 nCov-19 vaccine. Nature-portfolio [preprint]. [CrossRef]
- Kumar R, Singh N, Gautam S, Singh OP, Gidwani K, et al. (2014) Leishmania Specific CD4 T Cells Release IFNγ That Limits Parasite Replication in Patients with Visceral Leishmaniasis. PLOS Neglected Tropical Diseases 8(10): e3198. [CrossRef]
- Kumar, R., Bunn, P. T., Singh, S. S., Ng, S. S., Montes de Oca, M., De Labastida Rivera, F., Chauhan, S. B., Singh, N., Faleiro, R. J., Edwards, C. L., Frame, T. C. M., Sheel, M., Austin, R. J., Lane, S. W., Bald, T., Smyth, M. J., Hill, G. R., Best, S. E., Haque, A., … Engwerda, C. R. (2020). Type I interferons suppress anti-parasitic immunity and can be targeted to improve treatment of visceral leishmaniasis. Cell Reports, 30(8). [CrossRef]
- Kunz, N., & Kemper, C. (2021). Complement Has Brains-Do Intracellular Complement and Immunometabolism Cooperate in Tissue Homeostasis and Behavior? Frontiers in immunology, 12, 629986. [CrossRef]
- Kuriyama, Y., Shimizu, A., Kanai, S. et al. Coordination of retrotransposons and type I interferon with distinct interferon pathways in dermatomyositis, systemic lupus erythematosus and autoimmune blistering disease. Sci Rep 11, 23146 (2021). [CrossRef]
- Kuriyama, Y., Shimizu, A., Kanai, S., Oikawa, D., Tokunaga, F., Tsukagoshi, H., & Ishikawa, O. (2021). The synchronized gene expression of retrotransposons and type I interferon in dermatomyositis. Journal of the American Academy of Dermatology, 84(4), 1103–1105. [CrossRef]
- Kuroda, M., Halfmann, P. J., Hill-Batorski, L., Ozawa, M., Lopes, T., Neumann, G., Schoggins, J. W., Rice, C. M., & Kawaoka, Y. (2020). Identification of interferon-stimulated genes that attenuate Ebola virus infection. Nature communications, 11(1), 2953. [CrossRef]
- Kuypers F. A. (2022). Hyperinflammation, apoptosis, and organ damage. Experimental biology and medicine (Maywood, N.J.), 247(13), 1112–1123. [CrossRef]
- Kuzmin, I. V., Schwarz, T. M., Ilinykh, P. A., Jordan, I., Ksiazek, T. G., Sachidanandam, R., Basler, C. F., & Bukreyev, A. (2017). Innate Immune Responses of Bat and Human Cells to Filoviruses: Commonalities and Distinctions. Journal of virology, 91(8), e02471-16. [CrossRef]
- Kwon, J. S., Kim, J. Y., Kim, M. C., Park, S. Y., Kim, B. N., Bae, S., Cha, H. H., Jung, J., Kim, M. J., Lee, M. J., Choi, S. H., Chung, J. W., Shin, E. C., & Kim, S. H. (2020). Factors of Severity in Patients with COVID-19: Cytokine/Chemokine Concentrations, Viral Load, and Antibody Responses. The American journal of tropical medicine and hygiene, 103(6), 2412–2418. [CrossRef]
- La Bonnardière, C., Lefèvre, F., & Charley, B. (1994). Interferon response in pigs: molecular and biological aspects. Veterinary immunology and immunopathology, 43(1-3), 29–36. [CrossRef]
- Lagunas-Rangel, F. A., & Chávez-Valencia, V. (2020). High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. Journal of medical virology, 92(10), 1789–1790. [CrossRef]
- LaSalle, T. J., Gonye, A. L. K., Freeman, S. S., Kaplonek, P., Gushterova, I., Kays, K. R., Manakongtreecheep, K., Tantivit, J., Rojas-Lopez, M., Russo, B. C., Sharma, N., Thomas, M. F., Lavin-Parsons, K. M., Lilly, B. M., Mckaig, B. N., Charland, N. C., Khanna, H. K., Lodenstein, C. L., Margolin, J. D., Blaum, E. M., … Sade-Feldman, M. (2021). Longitudinal characterization of circulating neutrophils uncovers distinct phenotypes associated with disease severity in hospitalized COVID-19 patients. bioRxiv : the preprint server for biology, 2021.10.04.463121. [CrossRef]
- Lasfar, A., Abushahba, W., Balan, M., & Cohen-Solal, K. A. (2011). Interferon lambda: a new sword in cancer immunotherapy. Clinical & developmental immunology, 2011, 349575. [CrossRef]
- Lasfar, A., de laTorre, A., Abushahba, W., Cohen-Solal, K. A., Castaneda, I., Yuan, Y., Reuhl, K., Zloza, A., Raveche, E., Laskin, D. L., & Kotenko, S. V. (2016). Concerted action of IFN-α and IFN-λ induces local NK cell immunity and halts cancer growth. Oncotarget, 7(31), 49259–49267. [CrossRef]
- Lasfar, A., Gogas, H., Zloza, A., Kaufman, H. L., & Kirkwood, J. M. (2016). IFN-λ cancer immunotherapy: new kid on the block. Immunotherapy, 8(8), 877–888. [CrossRef]
- Lasfar, A., Zloza, A., & Cohen-Solal, K. A. (2016). IFN-lambda therapy: current status and future perspectives. Drug discovery today, 21(1), 167–171. [CrossRef]
- Laskovs, M., Partridge, L., & Slack, C. (2022). Molecular inhibition of RAS signalling to target ageing and age-related health. Disease models & mechanisms, 15(10), dmm049627. [CrossRef]
- Laviada-Molina, H. A., Leal-Berumen, I., Rodriguez-Ayala, E., & Bastarrachea, R. A. (2020). Working Hypothesis for Glucose Metabolism and SARS-CoV-2 Replication: Interplay Between the Hexosamine Pathway and Interferon RF5 Triggering Hyperinflammation. Role of BCG Vaccine?. Frontiers in endocrinology, 11, 514. [CrossRef]
- Lazear, H. M., Schoggins, J. W., & Diamond, M. S. (2019). Shared and Distinct Functions of Type I and Type III Interferons. Immunity, 50(4), 907–923. [CrossRef]
- Le Coupanec, A., Desforges, M., Kaufer, B., Dubeau, P., Côté, M., & Talbot, P. J. (2021). Potential differences in cleavage of the S protein and type-1 interferon together control human coronavirus infection, propagation, and neuropathology within the central nervous system. Journal of virology, 95(10), e00140-21. Advance online publication. [CrossRef]
- Le, H., Spearman, P., Waggoner, S. N., & Singh, K. (2022). Ebola virus protein VP40 stimulates IL-12- and IL-18-dependent activation of human natural killer cells. JCI insight, 7(16), e158902. [CrossRef]
- Lee AJ, Mian F, Poznanski SM, Stackaruk M, Chan T, Chew MV and Ashkar AA (2019) Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-γ Production. Front. Immunol 10, 1261. [CrossRef]
- Lee, J.S., Shin, EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol 20, 585–586 (2020). [CrossRef]
- Lee-Kirsch M. A. (2017). The Type I Interferonopathies. Annual review of medicine, 68, 297–315. [CrossRef]
- Lefèvre, F., Guillomot, M., D'Andréa, S., Battegay, S., & La Bonnardière, C. (1998). Interferon-delta: the first member of a novel type I interferon family. Biochimie, 80(8-9), 779–788. [CrossRef]
- Lekkerkerker, A. N., van Kooyk, Y., & Geijtenbeek, T. B. (2006). Viral piracy: HIV-1 targets dendritic cells for transmission. Current HIV research, 4(2), 169–176. [CrossRef]
- Letarov, A. V., Babenko, V. V., & Kulikov, E. E. (2021). Free SARS-CoV-2 Spike Protein S1 Particles May Play a Role in the Pathogenesis of COVID-19 Infection. Biochemistry. Biokhimiia, 86(3), 257–261. [CrossRef]
- Leung, L. W., Park, M. S., Martinez, O., Valmas, C., López, C. B., & Basler, C. F. (2011). Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunology and cell biology, 89(7), 792–802. [CrossRef]
- Levine, M. E., Lu, A. T., Quach, A., Chen, B. H., Assimes, T. L., Bandinelli, S., Hou, L., Baccarelli, A. A., Stewart, J. D., Li, Y., Whitsel, E. A., Wilson, J. G., Reiner, A. P., Aviv, A., Lohman, K., Liu, Y., Ferrucci, L., & Horvath, S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. [CrossRef]
- Li, B., Raghwani, J., Hill, S. C., Francois, S., Lefrancq, N., Liang, Y.,... & Tian, H. (2023). Association of poultry vaccination with the interspecies transmission and molecular evolution of H5 subtype avian influenza virus. bioRxiv, 2023-12. [CrossRef]
- Li, Q., Humphries, F., Girardin, R. C., Wallace, A., Ejemel, M., Amcheslavsky, A., McMahon, C. T., Schiller, Z. A., Ma, Z., Cruz, J., Dupuis, A. P., Payne, A. F., Maryam, A., Yilmaz, N. K., McDonough, K. A., Pierce, B. G., Schiffer, C. A., Kruse, A. C., Klempner, M. S., Cavacini, L. A., … Wang, Y. (2022). Mucosal nanobody IgA as inhalable and affordable prophylactic and therapeutic treatment against SARS-CoV-2 and emerging variants. Frontiers in immunology, 13, 995412. [CrossRef]
- Li, S.-fang, Gong, M.-jiao, Zhao, F.-rong, Shao, J.-jun, Xie, Y.-li, Zhang, Y.-guang, & Chang, H.-yun. (2018). Type I interferons: Distinct biological activities and current applications for viral infection. Cellular Physiology and Biochemistry, 51(5), 2377–2396. [CrossRef]
- Li, W., Wang, G., Zhang, H., Xin, G., Zhang, D., Zeng, J., Chen, X., Xu, Y., Cui, Y., & Li, K. (2010). Effects of NS1 variants of H5N1 influenza virus on interferon induction, TNFalpha response and p53 activity. Cellular & molecular immunology, 7(3), 235–242. [CrossRef]
- Li, X. , Li, Y., Fang, S., Su, J., Jiang, J., Liang, B., Huang, J., Zhou, B., Zang, N., Ho, W., Li, J., Li, Y., Chen, H., Ye, L., & Liang, H. (2017). Downregulation of autophagy-related gene ATG5 and GABARAP expression by IFN-λ1 contributes to its anti-HCV activity in human hepatoma cells. Antiviral research, 140, 83–94. [CrossRef]
- Li, X., Xie, J., Li, D., Li, H., Niu, Y., Wu, B., Yang, Y., Yan, Z., Zhang, X., Chen, L., & Feng, R. (2022). HSP27 Attenuates cGAS-Mediated IFN-β Signaling through Ubiquitination of cGAS and Promotes PRV Infection. Viruses, 14(9), 1851. [CrossRef]
- Li, Y., Li, C., Xue, P., Zhong, B., Mao, A. P., Ran, Y., Chen, H., Wang, Y. Y., Yang, F., & Shu, H. B. (2009). ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proceedings of the National Academy of Sciences of the United States of America, 106(19), 7945–7950. [CrossRef]
- Li, Z., Jiang, Y., Jiao, P., Wang, A., Zhao, F., Tian, G., Wang, X., Yu, K., Bu, Z., & Chen, H. (2006). The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. Journal of virology, 80(22), 11115–11123. [CrossRef]
- Lim, U., & Song, M. A. (2018). DNA Methylation as a Biomarker of Aging in Epidemiologic Studies. Methods in molecular biology (Clifton, N.J.), 1856, 219–231. [CrossRef]
- Lin, J., Cao, Y., Shah, A. U., Zuo, J., Zhang, S., Yu, Q., Chong, M., & Yang, Q. (2022). Inhibition of the antigen-presenting ability of dendritic cells by non-structural protein 2 of influenza A virus. Veterinary microbiology, 267, 109392. [CrossRef]
- Liu, J., Qian, C., & Cao, X. (2016). Post-Translational Modification Control of Innate Immunity. Immunity, 45(1), 15–30. [CrossRef]
- Liu, N., Pang, X., Zhang, H., & Ji, P. (2022). The cGAS-STING Pathway in Bacterial Infection and Bacterial Immunity. Frontiers in immunology, 12, 814709. [CrossRef]
- Liu, X. , Yin, L., Xue, M., Chen, J., Li, L., Fu, F., Feng, L., & Liu, P. (2022). Coronavirus Porcine Deltacoronavirus Upregulates MHC Class I Expression through RIG-I/IRF1-Mediated NLRC5 Induction. Journal of virology, 96(7), e0015822. [CrossRef]
- Locke, M., Lythe, G., López-García, M., Muñoz-Fontela, C., Carroll, M., & Molina-París, C. (2021). Quantification of Type I Interferon Inhibition by Viral Proteins: Ebola Virus as a Case Study. Viruses, 13(12), 2441. [CrossRef]
- Lodi, L. , Mastrolia, M. V., Bello, F., Rossi, G. M., Angelotti, M. L., Crow, Y. J., Romagnani, P., & Vaglio, A. (2022). Type I interferon-related kidney disorders. Kidney international, 101(6), 1142–1159. [CrossRef]
- Lopez, L., Sang, P. C., Tian, Y., & Sang, Y. (2020). Dysregulated Interferon Response Underlying Severe COVID-19. Viruses, 12(12), 1433. [CrossRef]
- Low, Z. Y., Zabidi, N. Z., Yip, A., Puniyamurti, A., Chow, V., & Lal, S. K. (2022). SARS-CoV-2 Non-Structural Proteins and Their Roles in Host Immune Evasion. Viruses, 14(9), 1991. [CrossRef]
- Lu, C. , Klement, J. D., Ibrahim, M. L., Xiao, W., Redd, P. S., Nayak-Kapoor, A., Zhou, G., & Liu, K. (2019). Type I interferon suppresses tumor growth through activating the STAT3-granzyme B pathway in tumor-infiltrating cytotoxic T lymphocytes. Journal for immunotherapy of cancer, 7(1), 157. [CrossRef]
- Lu, Y. , Cai, H., Lu, M., Ma, Y., Li, A., Gao, Y., Zhou, J., Gu, H., Li, J., & Gu, J. (2020). Porcine Epidemic Diarrhea Virus Deficient in RNA Cap Guanine-N-7 Methylation Is Attenuated and Induces Higher Type I and III Interferon Responses. Journal of virology, 94(16), e00447-20. [CrossRef]
- Lubaki, N. M., Younan, P., Santos, R. I., Meyer, M., Iampietro, M., Koup, R. A., & Bukreyev, A. (2016). The Ebola Interferon Inhibiting Domains Attenuate and Dysregulate Cell-Mediated Immune Responses. PLoS pathogens, 12(12), e1006031. [CrossRef]
- Luecke, S. , & Paludan, S. R. (2017). Molecular requirements for sensing of intracellular microbial nucleic acids by the innate immune system. Cytokine, 98, 4–14. [CrossRef]
- Luisetto, M. Luisetto, M., Almukthar, N., & Tarro, G., Intracellular Reverse Transcription of COVID-19 mRNA Vaccine, LAP LAMBERT Academic Publishing. 2022; 1, 3-67. 9: ISBN, 3157. [Google Scholar]
- Luo, K., Li, N., Ye, W., Gao, H., Luo, X., & Cheng, B. (2022). Activation of Stimulation of Interferon Genes (STING) Signal and Cancer Immunotherapy. Molecules (Basel, Switzerland), 27(14), 4638. [CrossRef]
- Lurie, N. , Saville, M., Hatchett, R., & Halton, J. (2020). Developing COVID-19 vaccines at pandemic speed. N Engl J Med, 382, 1969–1973. [CrossRef]
- Lv, L., Cao, M., Bai, J., Jin, L., Wang, X., Gao, Y., Liu, X., & Jiang, P. (2020). PRV-encoded UL13 protein kinase acts as an antagonist of innate immunity by targeting IRF3-signaling pathways. Veterinary microbiology, 250, 108860. [CrossRef]
- Lv, L., Cao, M., Bai, J., Jin, L., Wang, X., Gao, Y., Liu, X., & Jiang, P. (2020). PRV-encoded UL13 protein kinase acts as an antagonist of innate immunity by targeting IRF3-signaling pathways. Veterinary microbiology, 250, 108860. [CrossRef]
- Lyons-Weiler, J. (2020). Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoim, 3, 100051. [CrossRef]
- Lyudmila Shalamova, Ulrike Felgenhauer, Andreas R. Schaubmar, Kathrin Buettner, Marek Widera, Sandra Ciesek, Friedemann Weber (2022), Omicron variant of SARS-CoV-2 exhibits an increased resilience to the antiviral type I interferon response, doi:. [CrossRef]
- Ma, D. Y., & Suthar, M. S. (2015). Mechanisms of innate immune evasion in re-emerging RNA viruses. Current opinion in virology, 12, 26–37. [CrossRef]
- Ma, Y. , Su, X. Z., & Lu, F. (2020). The Roles of Type I Interferon in Co-infections With Parasites and Viruses, Bacteria, or Other Parasites. Frontiers in immunology, 11, 1805. [CrossRef]
- Ma, Y. , Wang, C., Xue, M., Fu, F., Zhang, X., Li, L., Yin, L., Xu, W., Feng, L., & Liu, P. (2018). The Coronavirus Transmissible Gastroenteritis Virus Evades the Type I Interferon Response through IRE1α-Mediated Manipulation of the MicroRNA miR-30a-5p/SOCS1/3 Axis. Journal of virology, 92(22), e00728-18. [CrossRef]
- Ma, Z. , Qin, Y., Jia, Y., Xie, Y., Qi, X., Guo, Y., He, J., Zhang, Y., Li, F., Yu, J., Zhu, H., Yang, F., Zhang, Y., Mao, R., & Zhang, J. (2022). Thyroid dysfunction incidence and risk factors in Chinese chronic hepatitis B patients treated with pegylated interferon alpha: A long-term follow-up study. Journal of viral hepatitis, 29(6), 412–419. [CrossRef]
- Magalhaes, J., Tresse, E., Ejlerskov, P., Hu, E., Liu, Y., Marin, A., Montalant, A., Satriano, L., Rundsten, C. F., Carlsen, E., Rydbirk, R., Sharifi-Zarchi, A., Andersen, J. B., Aznar, S., Brudek, T., Khodosevich, K., Prinz, M., Perrier, J. M., Sharma, M., Gasser, T., … Issazadeh-Navikas, S. (2021). PIAS2-mediated blockade of IFN-β signaling: a basis for sporadic Parkinson disease dementia. Molecular psychiatry, 26(10), 6083–6099. [CrossRef]
- Mahalapbutr, P. Mahalapbutr, P., Kongtaworn, N., & Rungrotmongkol, T. (2020). Structural insight into the recognition of S-adenosyl-L-homocysteine and sinefungin in SARS-CoV-2 Nsp16/Nsp10 RNA cap 2′-O-Methyltransferase. Computational and structural biotechnology journal, 2765. [Google Scholar]
- Makrinioti, H., Bush, A., Gern, J., Johnston, S. L., Papadopoulos, N., Feleszko, W., Camargo, C. A., Jr, Hasegawa, K., & Jartti, T. (2021). The Role of Interferons in Driving Susceptibility to Asthma Following Bronchiolitis: Controversies and Research Gaps. Frontiers in immunology, 12, 761660. Coomes, E. A., & Haghbayan, H. (2020). Frontiers in immunology, 12, 761660. [CrossRef]
- Malik, G., & Zhou, Y. (2020). Innate Immune Sensing of Influenza A Virus. Viruses, 12(7), 755. [CrossRef]
- Malmgaard L. (2004). Induction and regulation of IFNs during viral infections. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 24(8), 439–454. [CrossRef]
- Malur, M., Gale, M., Jr, & Krug, R. M. (2012). LGP2 downregulates interferon production during infection with seasonal human influenza A viruses that activate interferon regulatory factor 3. Journal of virology, 86(19), 10733–10738. [CrossRef]
- Manan, A., Pirzada, R. H., Haseeb, M., & Choi, S. (2022). Toll-like Receptor Mediation in SARS-CoV-2: A Therapeutic Approach. International journal of molecular sciences, 23(18), 10716. [CrossRef]
- Mangia, A., Mottola, L., & Santoro, R. (2013). Interleukin 28B polymorphisms as predictor of response in hepatitis C virus genotype 2 and 3 infected patients. World journal of gastroenterology, 19(47), 8924–8928. [CrossRef]
- Margolis, S. R., Wilson, S. C., & Vance, R. E. (2017). Evolutionary Origins of cGAS-STING Signaling. Trends in immunology, 38(10), 733–743. [CrossRef]
- Marquis, K. A., Becker, R. L., Weiss, A. N., Morris, M. C., & Ferran, M. C. (2020). The VSV matrix protein inhibits NF-κB and the interferon response independently in mouse L929 cells. Virology, 548, 117–123. [CrossRef]
- Marsh, G. A., & Wang, L. F. (2012). Hendra and Nipah viruses: why are they so deadly?. Current opinion in virology, 2(3), 242–247. [CrossRef]
- Martel, J., Ko, Y. F., Young, J. D., & Ojcius, D. M. (2020). Could nasal nitric oxide help to mitigate the severity of COVID-19? Microbes and infection, 22(4-5), 168–171. [CrossRef]
- Martins, D. , Dipasquale, O., Davies, K., Cooper, E., Tibble, J., Veronese, M., Frigo, M., Williams, S., Turkheimer, F., Cercignani, M., & Harrison, N. A. (2022). Transcriptomic and cellular decoding of functional brain connectivity changes reveal regional brain vulnerability to pro- and anti-inflammatory therapies. Brain, behavior, and immunity, 102, 312–323. [CrossRef]
- Mary Hongying Cheng, She Zhang, Rebecca A. Porritt, Magali Noval Rivas, Lisa Paschold, Edith Willscher, Mascha Binder, Mosche Arditi and Ivet Bahar (2020), Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation, Proceedings of the National Academy of Sciences of the United States of America, 117(41), pp 25254-25262, doi:. [CrossRef]
- Mateo, M., Reid, S. P., Leung, L. W., Basler, C. F., & Volchkov, V. E. (2010). Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. Journal of virology, 84(2), 1169–1175. [CrossRef]
- Mathern, D. R., & Heeger, P. S. (2015). Molecules Great and Small: The Complement System. Clinical journal of the American Society of Nephrology : CJASN, 10(9), 1636–1650. [CrossRef]
- Mathieu, E. , Ritchie, H., Ortiz-Ospina, E., Roser, M., Hasell, J., Appel, C., Giattino, C., & Ortiz-Ospina, E. (2021). A global database of COVID-19 vaccinations. Nat Hum Behav 5, 947–953, Data on COVID-19 (coronavirus) vaccinations by Our World in Data, accessible: https://github.com/owid/COVID-19-data/tree/master/public/data/vaccinations. [CrossRef]
- Matic, S. Matic, S., Popovic, S., Djurdjevic, P., Todorovic, D., Djordjevic, N., Mijailovic, Z.,... & Baskic, D. (2020). SARS-CoV-2 infection induces mixed M1/M2 phenotype in circulating monocytes and alterations in both dendritic cell and monocyte subsets. PLoS One, 0241. [Google Scholar]
- Matos, P., Pereira, J., & Jordan, P. (2022). Targeting Cancer by Using Nanoparticles to Modulate RHO GTPase Signaling. Advances in experimental medicine and biology, 1357, 115–127. [CrossRef]
- McCullough, P. A., Kelly, R.J., Ruocco, G., Lerma, E., Tumlin, J., Wheelan, K.R., Katz, N., Lepor, N.E., Vijay, K., Carter, H., Singh, B., McCullough, S.P., Bhambi, B.K., Palazzuoli, A., De Ferrari, G.M., Milligan, G.P., Safder, T., Tecson, K.M., Wang, D.D., McKinnon, J.E., O'Neill, W.W., Zervos, M., & Risch, H.A. (2021). Pathophysiological basis and rationale for early outpatient treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med, 134(1):16-22. [CrossRef]
- McDonough, A., Lee, R. V., & Weinstein, J. R. (2017). Microglial Interferon Signaling and White Matter. Neurochemical research, 42(9), 2625–2638. [CrossRef]
- McKellar, J., Rebendenne, A., Wencker, M., Moncorgé, O., & Goujon, C. (2021). Mammalian and Avian Host Cell Influenza A Restriction Factors. Viruses, 13(3), 522. [CrossRef]
- McNab, F. , Mayer-Barber, K., Sher, A., Wack, A., & O'Garra, A. (2015). Type I interferons in infectious disease. Nature reviews. Immunology, 15(2), 87–103. [CrossRef]
- Meng Zhongji, Wang Tongyu, Chen Li, Chen Xinhe, Li Longti, Qin Xueqin, Li Hai *, Luo Jie *, The Effect of Recombinant Human Interferon Alpha Nasal Drops to Prevent COVID-19 Pneumonia for Medical Staff in an Epidemic Area, Current Topics in Medicinal Chemistry 2021; 21(10). d.
- Menni, C. , Klaser, K., May, A., Polidori, L., Nguzen, L.H., Drew, D.A., Merino, J., Hu, C., Selvachandran, S., Antonelli, M., Murray, B., Canas, L.S., Molteni, E., Graham, M.S., Modat, M., Joshi, A.D., Mangino, M., Hammers, A., Goodman, A.L., Chan, A.T., Wolf, J., Steves, C.J., Valdes, A.M., Ourselin, S., & Spector, T.D. (2021). Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis, 21(7): 939-949. [CrossRef]
- Mesev, E. V., LeDesma, R. A., & Ploss, A. (2019). Decoding type I and III interferon signalling during viral infection. Nature microbiology, 4(6), 914–924. [CrossRef]
- Mesquita, S. D., Ferreira, A. C., Gao, F., Coppola, G., Geschwind, D. H., Sousa, J. C., Correia-Neves, M., Sousa, N., Palha, J. A., & Marques, F. (2015). The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer's disease. Brain, behavior, and immunity, 49, 280–292. [CrossRef]
- Mestecky J. (1987). The common mucosal immune system and current strategies for induction of immune responses in external secretions. Journal of clinical immunology, 7(4), 265–276. [CrossRef]
- Mestecky, J., & McGhee, J. R. (1992). Prospects for human mucosal vaccines. Advances in experimental medicine and biology, 327, 13–23. [CrossRef]
- Metz-Zumaran, C. , Kee, C., Doldan, P., Guo, C., Stanifer, M. L., & Boulant, S. (2022). Increased Sensitivity of SARS-CoV-2 to Type III Interferon in Human Intestinal Epithelial Cells. Journal of virology, 96(7), e0170521. [CrossRef]
- Meyer, K., Patra, T., Vijayamahantesh, & Ray, R. (2021). SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells. Journal of virology, 95(17), e0079421. [CrossRef]
- Meylan, P. R., Guatelli, J. C., Munis, J. R., Richman, D. D., & Kornbluth, R. S. (1993). Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology, 193(1), 138–148. [CrossRef]
- Michael W. Russell, Zina Moldoveanu, Pearay L. Ogra and Jiri Mesteacky, Mucosal Immunity in COVID- 19. [CrossRef]
- Minich, D. M., Henning, M., Darley, C., Fahoum, M., Schuler, C. B., & Frame, J. (2022). Is Melatonin the "Next Vitamin D"?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients, 14(19), 3934. [CrossRef]
- Mizutani, T., Fukushi, S., Saijo, M., Kurane, I., & Morikawa, S. (2004). Importance of Akt signaling pathway for apoptosis in SARS-CoV-infected Vero E6 cells. Virology, 327(2), 169–174. [CrossRef]
- Mo, S., Tang, W., Xie, J., Chen, S., Ren, L., Zang, N., Xie, X., Deng, Y., Gao, L., & Liu, E. (2021). Respiratory syncytial virus activates rab5a to suppress IRF1-dependent lambda interferon production, subverting the antiviral defense of airway epithelial cells. Journal of Virology, 95(8). [CrossRef]
- Moerdyk-Schauwecker, M., Shah, N. R., Murphy, A. M., Hastie, E., Mukherjee, P., & Grdzelishvili, V. Z. (2013). Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology, 436(1), 221–234. [CrossRef]
- Moozhipurath, R.K., Kraft, L. & Skiera, B. Evidence of protective role of Ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci Rep 10, 17705 (2020). [CrossRef]
- Moozhipurath, R.K., Kraft, L. Association of lockdowns with the protective role of ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci Rep 11, 22851 (2021). [CrossRef]
- Mordstein, M., Kochs, G., Dumoutier, L., Renauld, J. C., Paludan, S. R., Klucher, K., & Staeheli, P. (2008). Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS pathogens, 4(9), e1000151. [CrossRef]
- Mordstein, M., Michiels, T., & Staeheli, P. (2010). What have we learned from the IL28 receptor knockout mouse? Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 30(8), 579–584. [CrossRef]
- Mordstein, M., Neugebauer, E., Ditt, V., Jessen, B., Rieger, T., Falcone, V., Sorgeloos, F., Ehl, S., Mayer, D., Kochs, G., Schwemmle, M., Günther, S., Drosten, C., Michiels, T., & Staeheli, P. (2010). Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. Journal of virology, 84(11), 5670–5677. [CrossRef]
- Moreno, L., Quereda, C., Moreno, A., Perez-Elías, M. J., Antela, A., Casado, J. L., Dronda, F., Mateos, M. L., Bárcena, R., & Moreno, S. (2004). Pegylated interferon alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS (London, England), 18(1), 67–73. [CrossRef]
- Morita, N., Tanaka, Y., Odkhuu, E., Naiki, Y., Komatsu, T., & Koide, N. (2020). Sendai virus V protein decreases nitric oxide production by inhibiting RIG-I signaling in infected RAW264.7 macrophages. Microbes and infection, 22(8), 322–330. [CrossRef]
- Mortezaee, K., & Majidpoor, J. (2022). Cellular immune states in SARS-CoV-2-induced disease. Frontiers in Immunology, 13, doi:. [CrossRef]
- m: Cevik, Krutika Kuppalli, Jason Kindrachuk and Malik Peiris (2020), Virology, pathology and pathogenesis of SARS-CoV-2, BMJ, 371, 2020; -2. [CrossRef]
- Müller, L., Aigner, P., & Stoiber, D. (2017). Type I Interferons and Natural Killer Cell Regulation in Cancer. Frontiers in immunology, 8, 304. [CrossRef]
- Munir S, Hillyer P, Le Nouën C, Buchholz UJ, Rabin RL, et al. (2011) Respiratory Syncytial Virus Interferon Antagonist NS1 Protein Suppresses and Skews the Human T Lymphocyte Response. PLOS Pathogens 7(4): e1001336. [CrossRef]
- Murata, M., Nagai, M., Bando, S., Dobashi, H., & Takahara, J. (1993). Emergence of acute interstitial pneumonia following high dose interferon delta treatment in a case of chronic myelogenous leukemia. Internal medicine (Tokyo, Japan), 32(9), 716–718. [CrossRef]
- Murray, C.J.L. The Global Burden of Disease Study at 30 years. Nat Med 28, 2019–2026 (2022). [CrossRef]
- Mustelin, T., & Ukadike, K. C. (2020). How Retroviruses and Retrotransposons in Our Genome May Contribute to Autoimmunity in Rheumatological Conditions. Frontiers in immunology, 11, 593891. [CrossRef]
- Mustelin, T., & Ukadike, K. C. (2020). How Retroviruses and Retrotransposons in Our Genome May Contribute to Autoimmunity in Rheumatological Conditions. Frontiers in immunology, 11, 593891. [CrossRef]
- Myasnikov, A. L., Berns, S. A., Talyzin, P. A., & Ershov, F. I. (2021). Voprosy virusologii, 66(1), 47–54. [CrossRef]
- Naggie, S., Osinusi, A., Katsounas, A., Lempicki, R., Herrmann, E., Thompson, A. J., Clark, P. J., Patel, K., Muir, A. J., McHutchison, J. G., Schlaak, J. F., Trippler, M., Shivakumar, B., Masur, H., Polis, M. A., & Kottilil, S. (2012). Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: impaired viral kinetics and therapeutic response. Hepatology (Baltimore, Md.), 56(2), 444–454. [CrossRef]
- Naito, Y. , Takagi, T., Yamamoto, T., & Watanabe, S. (2020). Association between selective IgA deficiency and COVID-19. J Clin Biochem Nutr, 67(2): 122-125. [CrossRef]
- Narunsky-Haziza, L., Sepich-Poore, G. D., Livyatan, I., Asraf, O., Martino, C., Nejman, D., Gavert, N., Stajich, J. E., Amit, G., González, A., Wandro, S., Perry, G., Ariel, R., Meltser, A., Shaffer, J. P., Zhu, Q., Balint-Lahat, N., Barshack, I., Dadiani, M., … Straussman, R. (2022). Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell, 185(20), 3789–3806. [CrossRef]
- NatBiotech, (2016). Research not fit to print. Nat Biotechnol, 34(2): 115. [CrossRef]
- Navaratnarajah, C. K., Pease, D. R., Halfmann, P. J., Taye, B., Barkhymer, A., Howell, K. G., Charlesworth, J. E., Christensen, T. A., Kawaoka, Y., Cattaneo, R., Schneider, J. W., & Wanek Family Program for HLHS-Stem Cell Pipeline (2021). Highly Efficient SARS-CoV-2 Infection of Human Cardiomyocytes: Spike Protein-Mediated Cell Fusion and Its Inhibition. Journal of virology, 95(24), e0136821. [CrossRef]
- Nchioua, R. , Kmiec, D., Müller, J. A., Conzelmann, C., Groß, R., Swanson, C. M., Neil, S., Stenger, S., Sauter, D., Münch, J., Sparrer, K., & Kirchhoff, F. (2020). SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. mBio, 11(5), e01930-20. [CrossRef]
- Nelson, B. R., Roby, J. A., Dobyns, W. B., Rajagopal, L., Gale, M., Jr, & Adams Waldorf, K. M. (2020). Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain. Viral immunology, 33(1), 22–37. [CrossRef]
- Ngunjiri, J. M., Buchek, G. M., Mohni, K. N., Sekellick, M. J., & Marcus, P. I. (2013). Influenza virus subpopulations: exchange of lethal H5N1 virus NS for H1N1 virus NS triggers de novo generation of defective-interfering particles and enhances interferon-inducing particle efficiency. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 33(3), 99–107. [CrossRef]
- Nguyen, A. A., Habiballah, S. B., Platt, C. D., Geha, R. S., Chou, J. S., & McDonald, D. R. (2020). Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution! Clinical immunology (Orlando, Fla.), 216, 108459. [CrossRef]
- Nie, Y., Yang, D., & Oppenheim, J. J. (2016). Alarmins and Antitumor Immunity. Clinical therapeutics, 38(5), 1042–1053. [CrossRef]
- Nogales, A., Martinez-Sobrido, L., Topham, D. J., & DeDiego, M. L. (2017). NS1 Protein Amino Acid Changes D189N and V194I Affect Interferon Responses, Thermosensitivity, and Virulence of Circulating H3N2 Human Influenza A Viruses. Journal of virology, 91(5), e01930-16. [CrossRef]
- Nogales, A., Villamayor, L., Utrilla-Trigo, S., Ortego, J., Martinez-Sobrido, L., & DeDiego, M. L. (2021). Natural Selection of H5N1 Avian Influenza A Viruses with Increased PA-X and NS1 Shutoff Activity. Viruses, 13(9), 1760. [CrossRef]
- Odendall, C., & Kagan, J. C. (2015). The unique regulation and functions of type III interferons in antiviral immunity. Current opinion in virology, 12, 47–52. [CrossRef]
- Odkhuu, E., Komatsu, T., Naiki, Y., Koide, N., & Yokochi, T. (2014). Sendai virus C protein inhibits lipopolysaccharide-induced nitric oxide production through impairing interferon-β signaling. International immunopharmacology, 23(1), 267–272. [CrossRef]
- Odnokoz, O. , Yu, P., Peck, A. R., Sun, Y., Kovatich, A. J., Hooke, J. A., Hu, H., Mitchell, E. P., Rui, H., & Fuchs, S. Y. (2020). Malignant cell-specific pro-tumorigenic role of type I interferon receptor in breast cancers. Cancer biology & therapy, 21(7), 629–636. [CrossRef]
- Ogando, N. S., Zevenhoven-Dobbe, J. C., van der Meer, Y., Bredenbeek, P. J., Posthuma, C. C., & Snijder, E. J. (2020). The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2. Journal of virology, 94(23), e01246-20. [CrossRef]
- Oh, S. J., & Shin, O. S. (2021). SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response. Cells, 10(3), 530. [CrossRef]
- Oliva, A. , Kinter, A. L., Vaccarezza, M., Rubbert, A., Catanzaro, A., Moir, S., Monaco, J., Ehler, L., Mizell, S., Jackson, R., Li, Y., Romano, J. W., & Fauci, A. S. (1998). Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. The Journal of clinical investigation, 102(1), 223–231. [CrossRef]
- Olson, G. S., Murray, T. A., Jahn, A. N., Mai, D., Diercks, A. H., Gold, E. S., & Aderem, A. (2021). Type I interferon decreases macrophage energy metabolism during mycobacterial infection. Cell reports, 35(9), 109195. [CrossRef]
- Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T., & Seya, T. (2003). TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nature immunology, 4(2), 161–167. [CrossRef]
- Pabst R. (2015). Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)-Structure, function and species differences. Vaccine, 33(36), 4406–4413. [CrossRef]
- Padayachee, Y., Flicker, S., Linton, S., Cafferkey, J., Kon, O. M., Johnston, S. L., Ellis, A. K., Desrosiers, M., Turner, P., Valenta, R., & Scadding, G. K. (2021). Review: The Nose as a Route for Therapy. Part 2 Immunotherapy. Frontiers in allergy, 2, 668781. [CrossRef]
- Paidi, R. K., Jana, M., Mishra, R. K., Dutta, D., & Pahan, K. (2021). Selective Inhibition of the Interaction between SARS-CoV-2 Spike S1 and ACE2 by SPIDAR Peptide Induces Anti-Inflammatory Therapeutic Responses. Journal of immunology (Baltimore, Md. : 1950), 207(10), 2521–2533. [CrossRef]
- Paidi, R. K., Jana, M., Mishra, R. K., Dutta, D., Raha, S., & Pahan, K. (2021). ACE-2-interacting Domain of SARS-CoV-2 (AIDS) Peptide Suppresses Inflammation to Reduce Fever and Protect Lungs and Heart in Mice: Implications for COVID-19 Therapy. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 16(1), 59–70. [CrossRef]
- Paidi, R. K., Jana, M., Raha, S., McKay, M., Sheinin, M., Mishra, R. K., & Pahan, K. (2021). Eugenol, a Component of Holy Basil (Tulsi) and Common Spice Clove, Inhibits the Interaction Between SARS-CoV-2 Spike S1 and ACE2 to Induce Therapeutic Responses. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 16(4), 743–755. [CrossRef]
- Pan S, Manabe N, Yamaguchi Y (2021). 3D Structures of IgA, IgM, and Components. International Journal of Molecular Sciences; 22(23):12776. [CrossRef]
- Pantazi, I., Al-Qahtani, A. A., Alhamlan, F. S., Alothaid, H., Matou-Nasri, S., Sourvinos, G., Vergadi, E., & Tsatsanis, C. (2021). SARS-CoV-2/ACE2 Interaction Suppresses IRAK-M Expression and Promotes Pro-Inflammatory Cytokine Production in Macrophages. Frontiers in immunology, 12, 683800. [CrossRef]
- Paparisto, E., Hunt, N. R., Labach, D. S., Coleman, M. D., Di Gravio, E. J., Dodge, M. J., Friesen, N. J., Côté, M., Müller, A., Hoenen, T., & Barr, S. D. (2021). Interferon-Induced HERC5 Inhibits Ebola Virus Particle Production and Is Antagonized by Ebola Glycoprotein. Cells, 10(9), 2399. [CrossRef]
- Pardi N. , Weissman D. (2017) Nucleoside Modified mRNA Vaccines for Infectious Diseases. In: Kramps T., Elbers K. (eds) RNA Vaccines. Methods in Molecular Biology, vol 1499. Humana Press, New York, NY. [CrossRef]
- Park, A. , & Iwasaki, A. (2020). Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell host & microbe, 27(6), 870–878. [CrossRef]
- Park, G. J., Osinski, A., Hernandez, G., Eitson, J. L., Majumdar, A., Tonelli, M., Henzler-Wildman, K., Pawłowski, K., Chen, Z., Li, Y., Schoggins, J. W., & Tagliabracci, V. S. (2022). The mechanism of RNA capping by SARS-CoV-2. Nature, 609(7928), 793–800. [CrossRef]
- Pavel Kovarik, Castiglia Virginia, Ivin Masa, Ebner Florian (2016), Type I Interferons in Bacterial Infections: A Balancing Act, Frontiers in Immunology, Vol. 7, pp 652, doi: https://www.frontiersin.org/article/10. Frontiers in Immunology; //www: , Vol. 7, pp 652, doi: https, 3389. [Google Scholar]
- Peng, M. Y., Liu, W. C., Zheng, J. Q., Lu, C. L., Hou, Y. C., Zheng, C. M., Song, J. Y., Lu, K. C., & Chao, Y. C. (2021). Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. International journal of molecular sciences, 22(10), 5251. [CrossRef]
- Pereda, R., González, D., Rivero, H. B., Rivero, J. C., Pérez, A., López, L., Mezquia, N., Venegas, R., Betancourt, J. R., & Domínguez, R. E. (2020). Therapeutic Effectiveness of Interferon-α2b Against COVID-19: The Cuban Experience. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 40(9), 438–442. [CrossRef]
- Perwitasari, O., Cho, H., Diamond, M. S., & Gale, M., Jr (2011). Inhibitor of κB kinase epsilon (IKK(epsilon)), STAT1, and IFIT2 proteins define novel innate immune effector pathway against West Nile virus infection. The Journal of biological chemistry, 286(52), 44412–44423. [CrossRef]
- Pestka, S., Krause, C. D., & Walter, M. R. (2004). Interferons, interferon-like cytokines, and their receptors. Immunological reviews, 202, 8–32. [CrossRef]
- Phillpotts, R. J., Scott, G. M., Higgins, P. G., Wallace, J., Tyrrell, D. A., & Gauci, C. L. (1983). An effective dosage regimen for prophylaxis against rhinovirus infection by intranasal administration of HuIFN-alpha 2. Antiviral research, 3(2), 121–136. [CrossRef]
- Pierangeli, A., Gentile, M., Oliveto, G., Frasca, F., Sorrentino, L., Matera, L., Nenna, R., Viscido, A., Fracella, M., Petrarca, L., D'Ettorre, G., Ceccarelli, G., Midulla, F., Antonelli, G., & Scagnolari, C. (2022). Comparison by Age of the Local Interferon Response to SARS-CoV-2 Suggests a Role for IFN-ε and -ω. Frontiers in immunology, 13, 873232. [CrossRef]
- Pierce, C. A., Preston-Hurlburt, P., Dai, Y., Aschner, C. B., Cheshenko, N., Galen, B., Garforth, S. J., Herrera, N. G., Jangra, R. K., Morano, N. C., Orner, E., Sy, S., Chandran, K., Dziura, J., Almo, S. C., Ring, A., Keller, M. J., Herold, K. C., & Herold, B. C. (2020). Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Science translational medicine, 12(564), eabd5487. [CrossRef]
- Pieretti, J. C., Rubilar, O., Weller, R. B., Tortella, G. R., & Seabra, A. B. (2021). Nitric oxide (NO) and nanoparticles - Potential small tools for the war against COVID-19 and other human coronavirus infections. Virus research, 291, 198202. [CrossRef]
- Planas, D., Saunders, N., Maes, P., Guivel-Benhassine, F., Planchais, C., Buchrieser, J., Bolland, W. H., Porrot, F., Staropoli, I., Lemoine, F., Péré, H., Veyer, D., Puech, J., Rodary, J., Baele, G., Dellicour, S., Raymenants, J., Gorissen, S., Geenen, C., Vanmechelen, B., … Schwartz, O. (2022). Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature, 602(7898), 671–675. [CrossRef]
- Poli, G. , Biswas, P., & Fauci, A. S. (1994). Interferons in the pathogenesis and treatment of human immunodeficiency virus infection. Antiviral research, 24(2-3), 221–233. [CrossRef]
- Pons, J. C., Lebon, P., Frydman, R., & Delfraissy, J. F. (1995). Pharmacokinetics of interferon-alpha in pregnant women and fetoplacental passage. Fetal diagnosis and therapy, 10(1), 7–10. [CrossRef]
- Posch, W., Bermejo-Jambrina, M., Lass-Flörl, C., & Wilflingseder, D. (2020). Role of Complement Receptors (CRs) on DCs in Anti-HIV-1 Immunity. Frontiers in immunology, 11, 572114. [CrossRef]
- Posch, W., Bermejo-Jambrina, M., Steger, M., Witting, C., Diem, G., Hörtnagl, P., Hackl, H., Lass-Flörl, C., Huber, L. A., Geijtenbeek, T., & Wilflingseder, D. (2021). Complement Potentiates Immune Sensing of HIV-1 and Early Type I Interferon Responses. mBio, 12(5), e0240821. [CrossRef]
- Prabhakara, C., Godbole, R., Sil, P., Jahnavi, S., Gulzar, S. E., van Zanten, T. S., Sheth, D., Subhash, N., Chandra, A., Shivaraj, A., Panikulam, P., U, I., Nuthakki, V. K., Puthiyapurayil, T. P., Ahmed, R., Najar, A. H., Lingamallu, S. M., Das, S., Mahajan, B., Vemula, P., … Mayor, S. (2021). Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors. PLoS pathogens, 17(7), e1009706. [CrossRef]
- Prieto-Dominguez, N., Parnell, C., & Teng, Y. (2019). Drugging the Small GTPase Pathways in Cancer Treatment: Promises and Challenges. Cells, 8(3), 255. [CrossRef]
- Prokop, J. W., Hartog, N. L., Chesla, D., Faber, W., Love, C. P., Karam, R., Abualkheir, N., Feldmann, B., Teng, L., McBride, T., Leimanis, M. L., English, B. K., Holsworth, A., Frisch, A., Bauss, J., Kalpage, N., Derbedrossian, A., Pinti, R. M., Hale, N., Mills, J., … Rajasekaran, S. (2021). High-Density Blood Transcriptomics Reveals Precision Immune Signatures of SARS-CoV-2 Infection in Hospitalized Individuals. Frontiers in immunology, 12, 694243. [CrossRef]
- Puhl, A. C., Gomes, G. F., Damasceno, S., Fritch, E. J., Levi, J. A., Johnson, N. J., Scholle, F., Premkumar, L., Hurst, B. L., Lee-Montiel, F., Veras, F. P., Batah, S. S., Fabro, A. T., Moorman, N. J., Yount, B. L., Dickmander, R. J., Baric, R. S., Pearce, K. H., Cunha, F. Q., Alves-Filho, J. C., … Ekins, S. (2022). Vandetanib Blocks the Cytokine Storm in SARS-CoV-2-Infected Mice. ACS omega, 7(36), 31935–31944. [CrossRef]
- Qi, Y. Y., Zhou, X. J., Cheng, F. J., Hou, P., Ren, Y. L., Wang, S. X., Zhao, M. H., Yang, L., Martinez, J., & Zhang, H. (2018). Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-α in lupus nephritis. Annals of the rheumatic diseases, 77(12), 1799–1809. [CrossRef]
- Qiong Zhou, Virginia Chen, et al. (2020), “Interferon-a2b Treatment for COVID-19,” Frontiers in Immunology, Vol 11, Article 1601, doi:. [CrossRef]
- Quinones, Q. J., de Ridder, G. G., & Pizzo, S. V. (2008). GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histology and histopathology, 23(11), 1409–1416. [CrossRef]
- Radim Nencka, Jan Silhan, Martin Klima, Tomas Otava, Hugo Kocek, Petra Krafcikova, Evzen Boura (2022), Coronaviral RNA-methyltransferases: function, structure and inhibition, Nucleic Acids Research, 50(2), 635–650, https://doi.org/10. Nucleic Acids Research; //doi: , 50(2), 635–650, https, 1093. [Google Scholar]
- Radzikowska, U., Ding, M., Tan, G., Zhakparov, D., Peng, Y., Wawrzyniak, P., Wang, M., Li, S., Morita, H., Altunbulakli, C., Reiger, M., Neumann, A. U., Lunjani, N., Traidl-Hoffmann, C., Nadeau, K. C., O'Mahony, L., Akdis, C., & Sokolowska, M. (2020). Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy, 75(11), 2829–2845. [CrossRef]
- Raghavan, S., Kenchappa, D. B., & Leo, M. D. (2021, May 19). SARS-COV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity. Frontiers. Retrieved December 5, 2022, from https://www.frontiersin.org/articles/10. SARS-COV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity; //www: Frontiers. Retrieved December 5, 2022, from https, 3389. [Google Scholar]
- Rah, B., Rather, R. A., Bhat, G. R., Baba, A. B., Mushtaq, I., Farooq, M., Yousuf, T., Dar, S. B., Parveen, S., Hassan, R., Mohammad, F., Qassim, I., Bhat, A., Ali, S., Zargar, M. H., & Afroze, D. (2022). JAK/STAT Signaling: Molecular Targets, Therapeutic Opportunities, and Limitations of Targeted Inhibitions in Solid Malignancies. Frontiers in pharmacology, 13, 821344. [CrossRef]
- Ramasamy, S., & Subbian, S. (2021). Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clinical microbiology reviews, 34(3), e00299-20. [CrossRef]
- Rashid, F., Xie, Z., Suleman, M., Shah, A., Khan, S., & Luo, S. (2022). Roles and functions of SARS-CoV-2 proteins in host immune evasion. Frontiers in immunology, 13, 940756. [CrossRef]
- Rashid, F., Xie, Z., Suleman, M., Shah, A., Khan, S., & Luo, S. (2022). Roles and functions of SARS-CoV-2 proteins in host immune evasion. Frontiers in immunology, 13, 940756. [CrossRef]
- Ratajczak, M. Z., & Kucia, M. (2020). SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine "storm" and risk factor for damage of hematopoietic stem cells. Leukemia, 34(7), 1726–1729. [CrossRef]
- Rauchhaus, J., Robinson, J., Monti, L., & Di Antonio, M. (2022). G-quadruplexes Mark Sites of Methylation Instability Associated with Ageing and Cancer. Genes, 13(9), 1665. [CrossRef]
- Rautela, J., & Huntington, N. D. (2017). IL-15 signaling in NK cell cancer immunotherapy. Current opinion in immunology, 44, 1–6. [CrossRef]
- Rayner, J. O., Roberts, R. A., Kim, J., Poklepovic, A., Roberts, J. L., Booth, L., & Dent, P. (2020). AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochemical pharmacology, 182, 114227. [CrossRef]
- Reizis B. (2019). Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity, 50(1), 37–50. [CrossRef]
- Ren, Y., Cui, G., & Gao, Y. (2021). Research progress on inflammatory mechanism of primary Sjögren syndrome. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences, 50(6), 783–794. [CrossRef]
- Rezaie, A., Melmed, G.Y., Leite, G. et al. Endotracheal Application of Ultraviolet A Light in Critically Ill Patients with Severe Acute Respiratory Syndrome Coronavirus 2: A First-in-Human Study. Adv Ther 38, 4556–4568 (2021). [CrossRef]
- Richardson, G; recent developments and future strategies: , & Tate, B. (2000). Hormonal and pharmacological manipulation of the circadian clock.
- Ricke D. O. (2021). Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Frontiers in immunology, 12, 640093. [CrossRef]
- Rios, C. I., Cassatt, D. R., Hollingsworth, B. A., Satyamitra, M. M., Tadesse, Y. S., Taliaferro, L. P., Winters, T. A., & DiCarlo, A. L. (2021). Commonalities Between COVID-19 and Radiation Injury. Radiation research, 195(1), 1–24. [CrossRef]
- Robbins, M. A., Maksumova, L., Pocock, E., & Chantler, J. K. (2003). Nuclear factor-kappaB translocation mediates double-stranded ribonucleic acid-induced NIT-1 beta-cell apoptosis and up-regulates caspase-12 and tumor necrosis factor receptor-associated ligand (TRAIL). Endocrinology, 144(10), 4616–4625. [CrossRef]
- Rodriguez, J. J., Parisien, J. P., & Horvath, C. M. (2002). Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. Journal of virology, 76(22), 11476–11483. [CrossRef]
- Roers, A. , Hiller, B., & Hornung, V. (2016). Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity, 44(4), 739–754. [CrossRef]
- Romano, M., Ruggiero, A., Squeglia, F., Maga, G., & Berisio, R. (2020). A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells, 9(5), 1267. [CrossRef]
- Roskoski R., Jr (2016). Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacological research, 111, 784–803. [CrossRef]
- Roumenina, L. T., Daugan, M. V., Noé, R., Petitprez, F., Vano, Y. A., Sanchez-Salas, R., Becht, E., Meilleroux, J., Clec'h, B. L., Giraldo, N. A., Merle, N. S., Sun, C. M., Verkarre, V., Validire, P., Selves, J., Lacroix, L., Delfour, O., Vandenberghe, I., Thuilliez, C., Keddani, S., … Fridman, W. H. (2019). Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer immunology research, 7(7), 1091–1105. [CrossRef]
- Rubio-Casillas, A., Redwan, E. M., & Uversky, V. N. (2022). SARS-CoV-2: A Master of Immune Evasion. Biomedicines, 10(6), 1339. [CrossRef]
- Saadeldin, M. K., Abdel-Aziz, A. K., & Abdellatif, A. (2021). Dendritic cell vaccine immunotherapy; the beginning of the end of cancer and COVID-19. A hypothesis. Medical hypotheses, 146, 110365. [CrossRef]
- Safadi DE, Lebeau G, Lagrave A, Mélade J, Grondin L, Rosanaly S, Begue F, Hoareau M, Veeren B, Roche M, Hoarau J-J, Meilhac O, Mavingui P, Desprès P, Viranaïcken W, Krejbich-Trotot P. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses. 2023; 15(2):364. [CrossRef]
- Saito, H. Saito, H., Takenaka, H., Yoshida, S., Tsubokawa, T., Ogata, A., Imanishi, F., & Imanishi, J. (1985). Prevention from naturally acquired viral respiratory infection by interferon nasal spray. Rhinology.
- Saito, S., Ogino, T., Miyajima, N., Kato, A., & Kohase, M. (2002). Dephosphorylation failure of tyrosine-phosphorylated STAT1 in IFN-stimulated Sendai virus C protein-expressing cells. Virology, 293(2), 205–209. [CrossRef]
- Sakai, K., Ueda, A., Hasegawa, M., & Ueda, Y. (2018). Efficacy and safety of interferon alpha for essential thrombocythemia during pregnancy: two cases and a literature review. International journal of hematology, 108(2), 203–207. [CrossRef]
- Sakai, S., & Shichita, T. (2022). Role of alarmins in poststroke inflammation and neuronal repair. Seminars in immunopathology, 10.1007/s00281-022-00961-5. Advance online publication. [CrossRef]
- Salerno, F., Guislain, A., Cansever, D., & Wolkers, M. C. (2016). TLR-Mediated Innate Production of IFN-γ by CD8+ T Cells Is Independent of Glycolysis. Journal of immunology (Baltimore, Md. : 1950), 196(9), 3695–3705. [CrossRef]
- Saliu, T. P. Saliu, T. P., Umar, H. I., Ogunsile, O. J., Okpara, M. O., Yanaka, N., & Elekofehinti, O. O. (2021). Molecular docking and pharmacokinetic studies of phytocompounds from nigerian medicinal plants as promising inhibitory agents against SARS-CoV-2 methyltransferase (nsp16). Journal of Genetic Engineering and Biotechnology.
- Sampaio, N. G., Chauveau, L., Hertzog, J., Bridgeman, A., Fowler, G., Moonen, J. P., Dupont, M., Russell, R. A., Noerenberg, M., & Rehwinkel, J. (2021). The RNA sensor MDA5 detects SARS-CoV-2 infection. Scientific reports, 11(1), 13638. [CrossRef]
- Sánchez-Aparicio, M. T., Garcin, D., Rice, C. M., Kolakofsky, D., García-Sastre, A., & Baum, A. (2017). Loss of Sendai virus C protein leads to accumulation of RIG-I immunostimulatory defective interfering RNA. The Journal of general virology, 98(6), 1282–1293. [CrossRef]
- Sangaletti, P., Doe, J., Gatti, A., Arvay, C., Giuliani, L., & Lettner, H. (2022). SARS-CoV-2 and the Vaccination Hype. International Journal of Vaccine Theory, Practice, and Research, 2(1), 173–207. https://doi.org/10. International Journal of Vaccine Theory, Practice, and Research, 2; //doi: (1), 173–207. https, 5609. [Google Scholar] [CrossRef]
- Santer, D.M., Li, D., Ghosheh, Y. et al. Interferon-λ treatment accelerates SARS-CoV-2 clearance despite age-related delays in the induction of T cell immunity. Nat Commun 13, 6992 (2022). [CrossRef]
- Santerre, M., Arjona, S. P., Allen, C. N., Shcherbik, N., & Sawaya, B. E. (2021). Why do SARS-CoV-2 NSPs rush to the ER? Journal of neurology, 268(6), 2013–2022. [CrossRef]
- Santini, S. M., Lapenta, C., Santodonato, L., D'Agostino, G., Belardelli, F., & Ferrantini, M. (2009). IFN-alpha in the generation of dendritic cells for cancer immunotherapy. Handbook of experimental pharmacology, (188), 295–317. [CrossRef]
- Saramago, M., Bárria, C., Costa, V. G., Souza, C. S., Viegas, S. C., Domingues, S.,... & Matos, R. G. (2021). New targets for drug design: importance of nsp14/nsp10 complex formation for the 3’-5’exoribonucleolytic activity on SARS-CoV-2. The FEBS journal, 288(17), 5130-5147. [CrossRef]
- Satarker, S., Tom, A. A., Shaji, R. A., Alosious, A., Luvis, M., & Nampoothiri, M. (2021). JAK-STAT Pathway Inhibition and their Implications in COVID-19 Therapy. Postgraduate medicine, 133(5), 489–507. [CrossRef]
- Savulescu, J. , Giubilini, A., & Danchin, M. (2021). Global Ethical Considerations Regarding Mandatory Vaccination in Children. The Journal of pediatrics, 231, 10–16. [CrossRef]
- Schaefer, A., Unterberger, C., Frankenberger, M., Lohrum, M., Staples, K. J., Werner, T., Stunnenberg, H., & Ziegler-Heitbrock, L. (2009). Mechanism of interferon-gamma mediated down-regulation of interleukin-10 gene expression. Molecular Immunology, 46(7), 1351–1359. [CrossRef]
- Schardey, J., Globig, A. M., Janssen, C., Hofmann, M., Manegold, P., Thimme, R., & Hasselblatt, P. (2019). Vitamin D Inhibits Pro-Inflammatory T Cell Function in Patients With Inflammatory Bowel Disease. Journal of Crohn's & colitis, 13(12), 1546–1557. [CrossRef]
- Schmidt, A. , Rothenfusser, S., & Hopfner, K. P. (2012). Sensing of viral nucleic acids by RIG-I: from translocation to translation. European journal of cell biology, 91(1), 78–85. [CrossRef]
- Schmidt, N., Lareau, C. A., Keshishian, H., Ganskih, S., Schneider, C., Hennig, T., Melanson, R., Werner, S., Wei, Y., Zimmer, M., Ade, J., Kirschner, L., Zielinski, S., Dölken, L., Lander, E. S., Caliskan, N., Fischer, U., Vogel, J., Carr, S. A., Bodem, J., … Munschauer, M. (2021). The SARS-CoV-2 RNA-protein interactome in infected human cells. Nature microbiology, 6(3), 339–353. [CrossRef]
- Schoenmaker, L., Witzigmann, D., Kulkarni, J. A., Verbeke, R., Kersten, G., Jiskoot, W., & Crommelin, D. (2021). mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. International journal of pharmaceutics, 601, 120586. [CrossRef]
- Schomacker, H., Hebner, R. M., Boonyaratanakornkit, J., Surman, S., Amaro-Carambot, E., Collins, P. L., & Schmidt, A. C. (2012). The C proteins of human parainfluenza virus type 1 block IFN signaling by binding and retaining Stat1 in perinuclear aggregates at the late endosome. PloS one, 7(2), e28382. [CrossRef]
- Schreiber G. (2020). The Role of Type I Interferons in the Pathogenesis and Treatment of COVID-19. Frontiers in immunology, 11, 595739. [CrossRef]
- Scott, L. J., & Perry, C. M. (2002). Interferon-alpha-2b plus ribavirin: a review of its use in the management of chronic hepatitis C. Drugs, 62(3), 507–556. [CrossRef]
- Sebők C, Walmsley S, Tráj P, Mackei M, Vörösházi J, et al. (2022) Immunomodulatory effects of chicken cathelicidin-2 on a primary hepatic cell co-culture model. PLOS ONE 17(10): e0275847. [CrossRef]
- Segreto, R., & Deigin, Y. (2021). The genetic structure of SARS-CoV-2 does not rule out a laboratory origin: SARS-COV-2 chimeric structure and furin cleavage site might be the result of genetic manipulation. BioEssays : news and reviews in molecular, cellular and developmental biology, 43(3), e2000240. [CrossRef]
- Seneff, S; //ijvtpr.com/index: , Nigh, G. (2021). Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. International Journal of Vaccine Theory, Practice, and Research, 2(1): 38-79. Retrieved from https.
- Seneff, S., Nigh, G., Kyriakopoulos, A. M., & McCullough, P. A. (2022). Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 164, 113008. [CrossRef]
- Setaro, A. C., & Gaglia, M. M. (2021). All hands on deck: SARS-CoV-2 proteins that block early anti-viral interferon responses. Current research in virological science, 2, 100015. [CrossRef]
- Sette, A., & Crotty, S. (2021). Adaptive immunity to SARS-CoV-2 and COVID-19. Cell, 184(4), 861–880. [CrossRef]
- Seya, T., Matsumoto, M., Ebihara, T., & Oshiumi, H. (2009). Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunological reviews, 227(1), 44–53. [CrossRef]
- Shabunin,S. ,Gritsyuk,V.,Vostroilova,G.,Shabanov,D.,Khokhlova,N. & Korchagina,A.(2022).Study of Mutagenic and Antitoxic Properties of Gentabiferon-B. Macedonian Veterinary Review,45(1) 79-87. [CrossRef]
- Shafiq, A., Zubair, F., Ambreen, A., Suleman, M., Yousafi, Q., Rasul Niazi, Z., Anwar, Z., Khan, A., Mohammad, A., & Wei, D. Q. (2022). Investigation of the binding and dynamic features of A.30 variant revealed higher binding of RBD for hACE2 and escapes the neutralizing antibody: A molecular simulation approach. Computers in biology and medicine, 146, 105574. [CrossRef]
- Shan, C., Miao, S., Liu, C. et al. Induction of macrophage pyroptosis-related factors by pathogenic E. coli high pathogenicity island (HPI) in Yunnan Saba pigs. BMC Vet Res 17, 114 (2021). [CrossRef]
- Shao, S. C., Wang, C.H., Chang, K.C., Hung, M.J., Chen, H.Y., & Liao, S.C. (2021). Guillain-Barré Syndrome Associated with COVID-19 Vaccination. Emerg Infect Dis, 27(12): 3175-3178. [CrossRef]
- Shaver, K. A., Croom-Perez, T. J., & Copik, A. J. (2021). Natural Killer Cells: The Linchpin for Successful Cancer Immunotherapy. Frontiers in immunology, 12, 679117. [CrossRef]
- Shaw, M. L., García-Sastre, A., Palese, P., & Basler, C. F. (2004). Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. Journal of virology, 78(11), 5633–5641. [CrossRef]
- Sheng, L., Chen, X., Wang, Q., Lyu, S., & Li, P. (2020). Interferon-α2b enhances survival and modulates transcriptional profiles and the immune response in melanoma patients treated with dendritic cell vaccines. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 125, 109966. [CrossRef]
- Shepherd, J., Brodin, H., Cave, C., Waugh, N., Price, A., & Gabbay, J. (2004). Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health technology assessment (Winchester, England), 8(39), iii–125. [CrossRef]
- Shepherd, J., Jones, J., Hartwell, D., Davidson, P., Price, A., & Waugh, N. (2007). Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health technology assessment (Winchester, England), 11(11), 1–iii. [CrossRef]
- Sherer, N. M., & Mothes, W. (2008). Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends in cell biology, 18(9), 414–420. [CrossRef]
- Shi, J., Zhou, J., Zhang, X., Hu, W., Zhao, J. F., Wang, S., Wang, F. S., & Zhang, J. Y. (2021). Single-Cell Transcriptomic Profiling of MAIT Cells in Patients With COVID-19. Frontiers in immunology, 12, 700152. [CrossRef]
- Shi, Z. , & Puyo, C.A. (2020). Acetylcysteine to Combat COVID- 19. [CrossRef]
- Shibabaw T, Molla MD, Teferi B, Ayelign B. Role of IFN and Complements System: Innate Immunity in SARS-CoV-2. J Inflamm Res. 2020; 13, 507–518. [CrossRef]
- Shibabaw, T., Molla, M. D., Teferi, B., & Ayelign, B. (2020). Role of IFN and Complements System: Innate Immunity in SARS-CoV-2. Journal of inflammation research, 13, 507–518. [CrossRef]
- Shimoyama, S. , Kanisawa, Y., Ono, K., Souri, M., & Ichinose, A. (2021). First and fatal case of autoimmune acquired factor XIII/13 deficiency after COVID-19/SARS-CoV-2 vaccination. Am J Hematol, 1–3. [CrossRef]
- Shin, J., Toyoda, S., Fukuhara, A., & Shimomura, I. (2022). GRP78, a Novel Host Factor for SARS-CoV-2: The Emerging Roles in COVID-19 Related to Metabolic Risk Factors. Biomedicines, 10(8), 1995. [CrossRef]
- Shin, J., Toyoda, S., Nishitani, S., Fukuhara, A., Kita, S., Otsuki, M., & Shimomura, I. (2021). Possible Involvement of Adipose Tissue in Patients With Older Age, Obesity, and Diabetes With SARS-CoV-2 Infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of Hyperinsulinemia Management in COVID-19. Diabetes, 70(12), 2745–2755. [CrossRef]
- Shirazi, Y. , & Pitha, P. M. (1993). Interferon alpha-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology, 193(1), 303–312. [CrossRef]
- Sidahmed, A. M., León, A. J., Bosinger, S. E., Banner, D., Danesh, A., Cameron, M. J., & Kelvin, D. J. (2012). CXCL10 contributes to p38-mediated apoptosis in primary T lymphocytes in vitro. Cytokine, 59(2), 433–441. [CrossRef]
- Singh, P. K., Kulsum, U., Rufai, S. B., Mudliar, S. R., & Singh, S. (2020). Mutations in SARS-CoV-2 Leading to Antigenic Variations in Spike Protein: A Challenge in Vaccine Development. Journal of laboratory physicians, 12(2), 154–160. [CrossRef]
- Skene, D. J. Skene, D. J., Deacon, S., & Arendt, J. (1996). Use of melatonin in circadian rhythm disorders and following phase shifts. Acta neurobiologiae experimentalis.
- Smith G. L. (2018). Vaccinia Virus Protein C6: A Multifunctional Interferon Antagonist. Advances in experimental medicine and biology, 1052, 1–7. [CrossRef]
- Smith, B. L., Chen, G., Wilke, C. O., & Krug, R. M. (2018). Avian Influenza Virus PB1 Gene in H3N2 Viruses Evolved in Humans To Reduce Interferon Inhibition by Skewing Codon Usage toward Interferon-Altered tRNA Pools. mBio, 9(4), e01222-18. [CrossRef]
- Smolensky, M. H., Sackett-Lundeen, L. L., & Portaluppi, F. (2015). Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiology international, 32(8), 1029–1048. [CrossRef]
- Sodano, F., Gazzano, E., Fruttero, R., & Lazzarato, L. (2022). NO in Viral Infections: Role and Development of Antiviral Therapies. Molecules (Basel, Switzerland), 27(7), 2337. [CrossRef]
- Sodeifian, F, Nikfarjam, M, Kian, N, Mohamed, K, Rezaei, N. The role of type I interferon in the treatment of COVID-19. J Med Virol. 2021; 94, 63. [CrossRef]
- Sodeifian, F., Nikfarjam, M., Kian, N., Mohamed, K., & Rezaei, N. (2022). The role of type I interferon in the treatment of COVID-19. Journal of medical virology, 94(1), 63–81. [CrossRef]
- Solórzano, A., Webby, R. J., Lager, K. M., Janke, B. H., García-Sastre, A., & Richt, J. A. (2005). Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. Journal of virology, 79(12), 7535–7543. [CrossRef]
- Solov'ev V. D. (1969). The results of controlled observations on the prophylaxis of influenza with interferon. Bulletin of the World Health Organization.
- Soy, M., Keser, G., Atagündüz, P., Tabak, F., Atagündüz, I., & Kayhan, S. (2020). Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clinical rheumatology, 39(7), 2085–2094. [CrossRef]
- Sperber, S. J., Levine, P. A., Innes, D. J., Mills, S. E., & Hayden, F. G. (1988). Tolerance and efficacy of intranasal administration of recombinant beta serine interferon in healthy adults. The Journal of infectious diseases, 158(1), 166–175. [CrossRef]
- Stanifer, M. L., Guo, C., Doldan, P., & Boulant, S. (2020). Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces. Frontiers in immunology, 11, 608645. [CrossRef]
- Stawicki SP. Could tracheo-bronchial ultraviolet C irradiation be a valuable adjunct in the management of severe COVID-19 pulmonary infections?. Int J Acad Med [serial online] 2020 [cited 2022 Feb 28]; 6, 156–8.
- Steinman, R. M., Granelli-Piperno, A., Pope, M., Trumpfheller, C., Ignatius, R., Arrode, G., Racz, P., & Tenner-Racz, K. (2003). The interaction of immunodeficiency viruses with dendritic cells. Current topics in microbiology and immunology, 276, 1–30. [CrossRef]
- Sträter, J., & Möller, P. (2004). TRAIL and viral infection. Vitamins and hormones, 67, 257–274. [CrossRef]
- Su, C., & Zheng, C. (2017). Herpes Simplex Virus 1 Abrogates the cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway via Its Virion Host Shutoff Protein, UL41. Journal of virology, 91(6), e02414-16. [CrossRef]
- Sugai, A. , Sato, H., Takayama, I., Yoneda, M., & Kai, C. (2017). Nipah and Hendra Virus Nucleoproteins Inhibit Nuclear Accumulation of Signal Transducer and Activator of Transcription 1 (STAT1) and STAT2 by Interfering with Their Complex Formation. Journal of virology, 91(21), e01136-17. [CrossRef]
- Sumi, T. , Nagahisa, Y., Matsuura, K., Sekikawa, M., Yamada, Y., Nakata, H., & Chiba, H. (2021). Lung squamous cell carcinoma with hemoptysis after vaccination with tozinameran (BNT162b2, Pfizer-BioNTech). Thorac Cancer, 12, 3072–3075.
- Sun, J., Tang, X., Bai, R., Liang, C., Zeng, L., Lin, H., Yuan, R., Zhou, P., Huang, X., Xiong, Q., Peng, J., Cui, F., Ke, B., Su, J., Liu, Z., Lu, J., Tian, J., Sun, R., & Ke, C. (2020). The kinetics of viral load and antibodies to SARS-CoV-2. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 26(12), 1690.e1–1690.e4. [CrossRef]
- Sun, X., Belser, J. A., & Maines, T. R. (2020). Adaptation of H9N2 Influenza Viruses to Mammalian Hosts: A Review of Molecular Markers. Viruses, 12(5), 541. [CrossRef]
- Sung, R. Y., Yin, J., Oppenheimer, S. J., Tam, J. S., & Lau, J. (1993). Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Archives of disease in childhood, 69(4), 440–442. [CrossRef]
- Swiecki, M., & Colonna, M. (2011). Type I interferons: diversity of sources, production pathways and effects on immune responses. Current opinion in virology, 1(6), 463–475. [CrossRef]
- Takaki, H., Oshiumi, H., Sasai, M., Kawanishi, T., Matsumoto, M., & Seya, T. (2009). Oligomerized TICAM-1 (TRIF) in the cytoplasm recruits nuclear BS69 to enhance NF-kappaB activation and type I IFN induction. European journal of immunology, 39(12), 3469–3476. [CrossRef]
- Tan, X. , Sun, L., Chen, J., & Chen, Z. J. (2018). Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids. Annual review of microbiology, 72, 447–478. [CrossRef]
- Tang, Y., Zhang, P., Liu, Q., Cao, L., & Xu, J. (2022). Pyroptotic Patterns in Blood Leukocytes Predict Disease Severity and Outcome in COVID-19 Patients. Frontiers in immunology, 13, 888661. [CrossRef]
- Tannock, G. A., Gillett, S. M., Gillett, R. S., Barry, R. D., Hensley, M. J., Herd, R., Reid, A. L., & Saunders, N. A. (1988). A study of intranasally administered interferon A (rIFN-alpha 2A) for the seasonal prophylaxis of natural viral infections of the upper respiratory tract in healthy volunteers. Epidemiology and infection, 101(3), 611–621. [CrossRef]
- Tannock, G. A., Gillett, S. M., Gillett, R. S., Barry, R. D., Hensley, M. J., Herd, R., Reid, A. L., & Saunders, N. A. (1988). A study of intranasally administered interferon A (rIFN-alpha 2A) for the seasonal prophylaxis of natural viral infections of the upper respiratory tract in healthy volunteers. Epidemiology and infection, 101(3), 611–621. [CrossRef]
- Tarasova O, Ivanov S, Filimonov DA, Poroikov V. Data and Text Mining Help Identify Key Proteins Involved in the Molecular Mechanisms Shared by SARS-CoV-2 and HIV-1. Molecules. 2020; 25(12):2944. [CrossRef]
- Taylor, M. D., Korth, M. J., & Katze, M. G. (1998). Interferon treatment inhibits the replication of simian immunodeficiency virus at an early stage: evidence for a block between attachment and reverse transcription. Virology, 241(1), 156–162. [CrossRef]
- Time Magazine (1980), The Big IF in Cancer, https://time. 6855.
- Time Magazine (2023), A One-Shot COVID-19 Treatment Shows Promise, https://time. 6254.
- Timothy P. Riley, Hui-Ting Chou, Ruozhen Hu, Krzysztof P. Bzymek, Ana R. Correia, Alexander C. Partin, Danqing Li, Danyang Gong, Zhulun Wang, Xinchao Yu, Paolo Manzanillo and Fernando Garces (2021), Enhancing the Prefusion Conformational Stability of SARS-CoV-2 Spike Protein Through Structure-Guided Design, Frontiers in Immunology, doi:. [CrossRef]
- Tintoré M. (2009). New options for early treatment of multiple sclerosis. Journal of the neurological sciences, 277 Suppl 1, S9–S11. [CrossRef]
- Tonutti, A. , Motta, F., Ceribelli, A., Isailovic, N., Selmi, C., & De Santis, M. (2022). Anti-MDA5 Antibody Linking COVID-19, Type I Interferon, and Autoimmunity: A Case Report and Systematic Literature Review. Frontiers in immunology, 13, 937667. [CrossRef]
- Tresse, E., Riera-Ponsati, L., Jaberi, E., Sew, W., Ruscher, K., & Issazadeh-Navikas, S. (2021). IFN-β rescues neurodegeneration by regulating mitochondrial fission via STAT5, PGAM5, and Drp1. The EMBO journal, 40(11), e106868. [CrossRef]
- Tsuji, R. F., Geba, G. P., Wang, Y., Kawamoto, K., Matis, L. A., & Askenase, P. W. (1997). Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity, and late T cell interferon gamma: a possible initiating role of B cells. The Journal of experimental medicine, 186(7), 1015–1026. [CrossRef]
- Tufan, A. B., Lazarow, K., Kolesnichenko, M., Sporbert, A., von Kries, J. P., & Scheidereit, C. (2022). tsg101 associates with parp1 and is essential for parylation and dna damage-induced nf-κb activation. The EMBO Journal. [CrossRef]
- Tunbak, H., Enriquez-Gasca, R., Tie, C., Gould, P. A., Mlcochova, P., Gupta, R. K., Fernandes, L., Holt, J., van der Veen, A. G., Giampazolias, E., Burns, K. H., Maillard, P. V., & Rowe, H. M. (2020). The HUSH complex is a gatekeeper of type I interferon through epigenetic regulation of LINE-1s. Nature communications, 11(1), 5387. [CrossRef]
- Uriu, K. , Kimura, I., Shirakawa, K., Takaori-Kondo, A., Nakada, T.A., Kaneda, A., The Genotype to Phenotype Japan (G2P-Japan) Consortium, Nakagawa, S., & Sato, K. (2021). Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera. bioRxiv, 2021.09.06.459005. [CrossRef]
- van den Berg, D. F., & Te Velde, A. A. (2020). Severe COVID-19: NLRP3 Inflammasome Dysregulated. Frontiers in immunology, 11, 1580. [CrossRef]
- Vanderheiden, A., Ralfs, P., Chirkova, T., Upadhyay, A. A., Zimmerman, M. G., Bedoya, S., Aoued, H., Tharp, G. M., Pellegrini, K. L., Manfredi, C., Sorscher, E., Mainou, B., Lobby, J. L., Kohlmeier, J. E., Lowen, A. C., Shi, P. Y., Menachery, V. D., Anderson, L. J., Grakoui, A., Bosinger, S. E., … Suthar, M. S. (2020). Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. Journal of virology, 94(19), e00985-20. [CrossRef]
- Vanderheiden, A. , Ralfs, P., Chirkova, T., Upadhyay, A. A., Zimmerman, M. G., Bedoya, S., Aoued, H., Tharp, G. M., Pellegrini, K. L., Manfredi, C., Sorscher, E., Mainou, B., Lobby, J. L., Kohlmeier, J. E., Lowen, A. C., Shi, P. Y., Menachery, V. D., Anderson, L. J., Grakoui, A., Bosinger, S. E., … Suthar, M. S. (2020). Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. Journal of virology, 94(19), e00985-20. [CrossRef]
- Vanderheiden, A., Ralfs, P., Chirkova, T., Upadhyay, A. A., Zimmerman, M. G., Bedoya, S., Aoued, H., Tharp, G. M., Pellegrini, K. L., Manfredi, C., Sorscher, E., Mainou, B., Lobby, J. L., Kohlmeier, J. E., Lowen, A. C., Shi, P. Y., Menachery, V. D., Anderson, L. J., Grakoui, A., Bosinger, S. E., … Suthar, M. S. (2020). Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. Journal of virology, 94(19), e00985-20. [CrossRef]
- Vandoorn, E., Stadejek, W., Parys, A., Chepkwony, S., Chiers, K., & Van Reeth, K. (2022). Pathobiology of an NS1-Truncated H3N2 Swine Influenza Virus Strain in Pigs. Journal of virology, 96(11), e0051922. [CrossRef]
- Varble, A. J., Ried, C. D., Hammond, W. J., Marquis, K. A., Woodruff, M. C., & Ferran, M. C. (2016). The vesicular stomatitis virus matrix protein inhibits NF-κB activation in mouse L929 cells. Virology, 499, 99–104. [CrossRef]
- Vidy, A., Chelbi-Alix, M., & Blondel, D. (2005). Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. Journal of virology, 79(22), 14411–14420. [CrossRef]
- Vignau, J. , Karila, L., Costisella, O., & Canva, V. (2005). Hépatite C, Interféron alpha et dépression: principales hypothèses physiopathologiques [Hepatitis C, interferon a and depression: main physiopathologic hypothesis]. L'Encephale, 31(3), 349–357. [CrossRef]
- Villarreal, L; Kolb V (ed): P. (2015) Virolution Can Help Us Understand the Origin of Life. In; ISBN 13-978-1-4665-8462-4.
- Villarreal, L. P., Witzany, G. (2021) Social networking of quasi-species consortia drive virolution via persistence. AIMS Microbiology, 7(2): 138-162. [CrossRef]
- Vishnubalaji, R. , Shaath, H., & Alajez, N. M. (2020). Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes, 11(7), 760. [CrossRef]
- Vital, E. M., Merrill, J. T., Morand, E. F., Furie, R. A., Bruce, I. N., Tanaka, Y., Manzi, S., Kalunian, K. C., Kalyani, R. N., Streicher, K., Abreu, G., & Tummala, R. (2022). Anifrolumab efficacy and safety by type I interferon gene signature and clinical subgroups in patients with SLE: post hoc analysis of pooled data from two phase III trials. Annals of the rheumatic diseases, 81(7), 951–961. [CrossRef]
- W. A. Robinson, T. I. Mughal, M. R. Thomas, Melinda Johnson, R. J. Spiegel (1986), Treatment of Metastatic Malignant Melanoma with Recombinant Interferon Alpha 2, Immunobiology, Vol 172, 3-5, pp 275-282,. [CrossRef]
- Wan, D., Jiang, W., & Hao, J. (2020). Research Advances in How the cGAS-STING Pathway Controls the Cellular Inflammatory Response. Frontiers in immunology, 11, 615. [CrossRef]
- Wang, C. , Shan, L., Qu, S., Xue, M., Wang, K., Fu, F., Wang, L., Wang, Z., Feng, L., Xu, W., & Liu, P. (2020). The Coronavirus PEDV Evades Type III Interferon Response Through the miR-30c-5p/SOCS1 Axis. Frontiers in microbiology, 11, 1180. [CrossRef]
- Wang, C., Wang, T., Duan, L., Chen, H., Hu, R., Wang, X., Jia, Y., Chu, Z., Liu, H., Wang, X., Zhang, S., Xiao, S., Wang, J., Dang, R., & Yang, Z. (2022). Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins. Frontiers in microbiology, 12, 790191. [CrossRef]
- Yao xue xue bao = Acta pharmaceutica Sinica, 49; //pubmed: (11), 1547–1553, available at: https.
- Yao xue xue bao = Acta pharmaceutica Sinica, 49; //pubmed: (11), 1547–1553, doi: https.
- Wang, M., Liu, Y., Qin, C., Lang, Y., Xu, A., Yu, C., Zhao, Z., Zhang, R., Yang, J., & Tang, J. (2022). Pseudorabies Virus EP0 Antagonizes the Type I Interferon Response via Inhibiting IRF9 Transcription. Journal of virology, 96(13), e0217121. [CrossRef]
- Wang, N., Zhan, Y., Zhu, L., Hou, Z., Liu, F., Song, P., Qiu, F., Wang, X., Zou, X., Wan, D., Qian, X., Wang, S., Guo, Y., Yu, H., Cui, M., Tong, G., Xu, Y., Zheng, Z., Lu, Y., & Hong, P. (2020). Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell host & microbe, 28(3), 455–464.e2. [CrossRef]
- Wang, Y., Luo, J., Alu, A., Han, X., Wei, Y., & Wei, X. (2020). cGAS-STING pathway in cancer biotherapy. Molecular cancer, 19(1), 136. [CrossRef]
- Wang, Y., Qian, G., Zhu, L., Zhao, Z., Liu, Y., Han, W., Zhang, X., Zhang, Y., Xiong, T., Zeng, H., Yu, X., Yu, X., Zhang, X., Xu, J., Zou, Q., & Yan, D. (2022). HIV-1 Vif suppresses antiviral immunity by targeting STING. Cellular & molecular immunology, 19(1), 108–121. [CrossRef]
- Wang, Y., Sun, Y., Wu, A., Xu, S., Pan, R., Zeng, C., Jin, X., Ge, X., Shi, Z., Ahola, T., Chen, Y., & Guo, D. (2015). Coronavirus nsp10/nsp16 Methyltransferase Can Be Targeted by nsp10-Derived Peptide In Vitro and In Vivo To Reduce Replication and Pathogenesis. Journal of virology, 89(16), 8416–8427. [CrossRef]
- Warfield, K. L., Perkins, J. G., Swenson, D. L., Deal, E. M., Bosio, C. M., Aman, M. J., Yokoyama, W. M., Young, H. A., & Bavari, S. (2004). Role of natural killer cells in innate protection against lethal ebola virus infection. The Journal of experimental medicine, 200(2), 169–179. [CrossRef]
- Webb, L. G., & Fernandez-Sesma, A. (2022). RNA viruses and the cGAS-STING pathway: reframing our understanding of innate immune sensing. Current opinion in virology, 53, 101206. [CrossRef]
- Wei, X., Xie, F., Zhou, X., Wu, Y., Yan, H., Liu, T., Huang, J., Wang, F., Zhou, F., & Zhang, L. (2022). Role of pyroptosis in inflammation and cancer. Cellular & molecular immunology, 19(9), 971–992. [CrossRef]
- West, E. E., Kunz, N., & Kemper, C. (2020). Complement and human T cell metabolism: Location, location, location. Immunological reviews, 295(1), 68–81. [CrossRef]
- Woo, J., Shin, S., Cho, E., Ryu, D., Garandeau, D., Chajra, H., Fréchet, M., Park, D., & Jung, E. (2021). Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PloS one, 16(12), e0260545. [CrossRef]
- Woo, S. R., Corrales, L., & Gajewski, T. F. (2015). Innate immune recognition of cancer. Annual review of immunology, 33, 445–474. [CrossRef]
- Woolsey, C., Menicucci, A. R., Cross, R. W., Luthra, P., Agans, K. N., Borisevich, V., Geisbert, J. B., Mire, C. E., Fenton, K. A., Jankeel, A., Anand, S., Ebihara, H., Geisbert, T. W., Messaoudi, I., & Basler, C. F. (2019). A VP35 Mutant Ebola Virus Lacks Virulence but Can Elicit Protective Immunity to Wild-Type Virus Challenge. Cell reports, 28(12), 3032–3046.e6. [CrossRef]
- Xia X. (2020). Extreme Genomic CpG Deficiency in SARS-CoV-2 and Evasion of Host Antiviral Defense. Molecular biology and evolution, 37(9), 2699–2705. [CrossRef]
- Xia, H., & Shi, P. Y. (2020). Antagonism of Type I Interferon by Severe Acute Respiratory Syndrome Coronavirus 2. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research, 40(12), 543–548. [CrossRef]
- Xia, H., Cao, Z., Xie, X., Zhang, X., Chen, J. Y., Wang, H., Menachery, V. D., Rajsbaum, R., & Shi, P. Y. (2020). Evasion of Type I Interferon by SARS-CoV-2. Cell reports, 33(1), 108234. [CrossRef]
- Xia, P., Wang, S., Gao, P., Gao, G., & Fan, Z. (2016). DNA sensor cGAS-mediated immune recognition. Protein & cell, 7(11), 777–791. [CrossRef]
- Xiang, Y., Wang, M., Chen, H., & Chen, L. (2021). Potential therapeutic approaches for the early entry of SARS-CoV-2 by interrupting the interaction between the spike protein on SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2). Biochemical pharmacology, 192, 114724. [CrossRef]
- Xing, F., Matsumiya, T., Shiba, Y., Hayakari, R., Yoshida, H., & Imaizumi, T. (2016). Non-Canonical Role of IKKα in the Regulation of STAT1 Phosphorylation in Antiviral Signaling. PloS one, 11(12), e0168696. [CrossRef]
- Xu, J. , Mercado-López, X., Grier, J. T., Kim, W. K., Chun, L. F., Irvine, E. B., Del Toro Duany, Y., Kell, A., Hur, S., Gale, M., Jr, Raj, A., & López, C. B. (2015). Identification of a Natural Viral RNA Motif That Optimizes Sensing of Viral RNA by RIG-I. mBio, 6(5), e01265-15. [CrossRef]
- Xu, L. , Li, M., Yang, Y., Zhang, C., Xie, Z., Tang, J., Shi, Z., Chen, S., Li, G., Gu, Y., Wang, X., Zhang, F., Wang, Y., & Shen, X. (2022). Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release. mBio, 13(3), e0363221. [CrossRef]
- Xu, Z., Chen, Y., & Chen, Y. (2019). Spatiotemporal Regulation of Rho GTPases in Neuronal Migration. Cells, 8(6), 568. [CrossRef]
- Xu, Z., Choi, J. H., Dai, D. L., Luo, J., Ladak, R. J., Li, Q., Wang, Y., Zhang, C., Wiebe, S., Liu, A. C. H., Ran, X., Yang, J., Naeli, P., Garzia, A., Zhou, L., Mahmood, N., Deng, Q., Elaish, M., Lin, R., Mahal, L. K., … Sonenberg, N. (2022). SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proceedings of the National Academy of Sciences of the United States of America, 119(32), e2204539119. [CrossRef]
- Xue, B., Li, H., Liu, S., Feng, Q., Xu, Y., Deng, R., Chen, S., Wang, J., Li, X., Wan, M., Tang, S., & Zhu, H. (2022). The redox cycling of STAT2 maintains innate immune homeostasis. Cell Reports, 40(7), 111215. [CrossRef]
- Yamada, T. , Sato, S., Sotoyama, Y., Orba, Y., Sawa, H., Yamauchi, H., Sasaki, M., & Takaoka, A. (2021). RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nature immunology, 22(7), 820–828. [CrossRef]
- Yang, Q. , Lin, F., Wang, Y., Zeng, M., & Luo, M. (2021). Long Noncoding RNAs as Emerging Regulators of COVID-19. Frontiers in immunology, 12, 700184. [CrossRef]
- Yang, Y., Jing, Y., Yang, J., & Yang, Q. (2018). Effects of intranasal administration with Bacillus subtilis on immune cells in the nasal mucosa and tonsils of piglets. Experimental and therapeutic medicine, 15(6), 5189–5198. [CrossRef]
- Yao, H. , Dittmann, M., Peisley, A., Hoffmann, H. H., Gilmore, R. H., Schmidt, T., Schmidt-Burgk, J., Hornung, V., Rice, C. M., & Hur, S. (2015). ATP-dependent effector-like functions of RIG-I-like receptors. Molecular cell, 58(3), 541–548. [CrossRef]
- Yazdani Brojeni, P., Matok, I., Garcia Bournissen, F., & Koren, G. (2012). A systematic review of the fetal safety of interferon alpha. Reproductive toxicology (Elmsford, N.Y.), 33(3), 265–268. [CrossRef]
- Yin, L. , Liu, X., Hu, D., Luo, Y., Zhang, G., & Liu, P. (2022). Swine Enteric Coronaviruses (PEDV, TGEV, and PDCoV) Induce Divergent Interferon-Stimulated Gene Responses and Antigen Presentation in Porcine Intestinal Enteroids. Frontiers in immunology, 12, 826882. [CrossRef]
- Yin, X. , Riva, L., Pu, Y., Martin-Sancho, L., Kanamune, J., Yamamoto, Y., Sakai, K., Gotoh, S., Miorin, L., De Jesus, P. D., Yang, C. C., Herbert, K. M., Yoh, S., Hultquist, J. F., García-Sastre, A., & Chanda, S. K. (2021). MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell reports, 34(2), 108628. [CrossRef]
- Yin, Y., Romero, N., & Favoreel, H. W. (2021). Pseudorabies Virus Inhibits Type I and Type III Interferon-Induced Signaling via Proteasomal Degradation of Janus Kinases. Journal of virology, 95(20), e0079321. [CrossRef]
- Yockey, L. J., Jurado, K. A., Arora, N., Millet, A., Rakib, T., Milano, K. M., Hastings, A. K., Fikrig, E., Kong, Y., Horvath, T. L., Weatherbee, S., Kliman, H. J., Coyne, C. B., & Iwasaki, A. (2018). Type I interferons instigate fetal demise after Zika virus infection. Science immunology, 3(19), eaao1680. [CrossRef]
- Yoo, J. S., Sasaki, M., Cho, S. X., Kasuga, Y., Zhu, B., Ouda, R., Orba, Y., de Figueiredo, P., Sawa, H., & Kobayashi, K. S. (2021). SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nature communications, 12(1), 6602. [CrossRef]
- Yoo, JS., Sasaki, M., Cho, S.X. et al. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat Commun 12, 6602 (2021). [CrossRef]
- Yoshida, A. , Kawabata, R., Honda, T., Sakai, K., Ami, Y., Sakaguchi, T., & Irie, T. (2018). A Single Amino Acid Substitution within the Paramyxovirus Sendai Virus Nucleoprotein Is a Critical Determinant for Production of Interferon-Beta-Inducing Copyback-Type Defective Interfering Genomes. Journal of virology, 92(5), e02094-17. [CrossRef]
- Yoshida, Y., Katsurada, T., Nakabou, Y., & Kawabata, H. (2017). Efficacy of interferon-alpha in essential thrombocythemia during pregnancy. Annals of hematology, 96(5), 877–878. [CrossRef]
- Zhonghua shi yan he lin chuang bing du xue za zhi = Zhonghua shiyan he linchuang bingduxue zazhi = Chinese journal of experimental and clinical virology, 19; //pubmed: (3), 216–219, doi: https.
- Yu, Q., Carbone, C. J., Katlinskaya, Y. V., Zheng, H., Zheng, K., Luo, M., Wang, P. J., Greenberg, R. A., & Fuchs, S. Y. (2015). Type I interferon controls propagation of long interspersed element-1. The Journal of biological chemistry, 290(16), 10191–10199. [CrossRef]
- Yu, R. , Zhu, B., & Chen, D. (2022). Type I interferon-mediated tumor immunity and its role in immunotherapy. Cellular and molecular life sciences : CMLS, 79(3), 191. [CrossRef]
- Yu, S., Ge, H., Li, S., & Qiu, H. J. (2022). Modulation of Macrophage Polarization by Viruses: Turning Off/On Host Antiviral Responses. Frontiers in microbiology, 13, 839585. [CrossRef]
- Yu, W., Wu, X., Zhao, Y., Chen, C., Yang, Z., Zhang, X., Ren, J., Wang, Y., Wu, C., Li, C., Chen, R., Wang, X., Zheng, W., Liao, H., & Yuan, X. (2021). Computational Simulation of HIV Protease Inhibitors to the Main Protease (Mpro) of SARS-CoV-2: Implications for COVID-19 Drugs Design. Molecules (Basel, Switzerland), 26(23), 7385. [CrossRef]
- Yuanlin Ma, Xin-Zhuan Su and Fangli Lu (2020), The Roles of Type I Interferon in Co-infections With Parasites and Viruses, Bacteria, or Other Parasites, Front. Immunol., doi:. [CrossRef]
- Yuen, C. K., Lam, J. Y., Wong, W. M., Mak, L. F., Wang, X., Chu, H., Cai, J. P., Jin, D. Y., To, K. K., Chan, J. F., Yuen, K. Y., & Kok, K. H. (2020). SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerging microbes & infections, 9(1), 1418–1428. [CrossRef]
- Zanoni, I., Granucci, F., & Broggi, A. (2017). Interferon (IFN)-λ Takes the Helm: Immunomodulatory Roles of Type III IFNs. Frontiers in immunology, 8, 1661. [CrossRef]
- Zhang L, Richards A, Khalil A, Wogram E, Ma H, Young RA, Jaenisch R. SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome. bioRxiv [Preprint]. 2020 Dec 13, 2020. [CrossRef]
- Zhang, C., Yang, Y., Zhou, X., Liu, X., Song, H., He, Y., & Huang, P. (2010). Highly pathogenic avian influenza A virus H5N1 NS1 protein induces caspase-dependent apoptosis in human alveolar basal epithelial cells. Virology journal, 7, 51. [CrossRef]
- Zhang, C., Yang, Y., Zhou, X., Yang, Z., Liu, X., Cao, Z., Song, H., He, Y., & Huang, P. (2011). The NS1 protein of influenza A virus interacts with heat shock protein Hsp90 in human alveolar basal epithelial cells: implication for virus-induced apoptosis. Virology journal, 8, 181. [CrossRef]
- Zhang, J. , Zhao, C., & Zhao, W. (2021). Virus Caused Imbalance of Type I IFN Responses and Inflammation in COVID-19. Frontiers in immunology, 12, 633769. [CrossRef]
- Zhang, J., Zhao, C., & Zhao, W. (2021). Virus Caused Imbalance of Type I IFN Responses and Inflammation in COVID-19. Frontiers in immunology, 12, 633769. [CrossRef]
- Zhang, M. , Zheng, S., & Liang, J. Q. (2022). Transcriptional and reverse transcriptional regulation of host genes by human endogenous retroviruses in cancers. Frontiers in microbiology, 13, 946296. [CrossRef]
- Zhang, Q. , & Yoo, D. (2016). Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus research, 226, 128–141. [CrossRef]
- Zhang, Q. , Ke, H., Blikslager, A., Fujita, T., & Yoo, D. (2018). Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling. Journal of virology, 92(4), e01677-17. [CrossRef]
- Zhang, Q., Wang, C., Ma, F., Yao, L., Gao, H., Zhu, L. and Zheng, L., 2020. Development and biological activity of long-acting recombinant human interferon-α2b. BMC biotechnology, 20, pp.1-9. [CrossRef]
- Zhang, R., Xu, A., Qin, C., Zhang, Q., Chen, S., Lang, Y., Wang, M., Li, C., Feng, W., Zhang, R., Jiang, Z., & Tang, J. (2017). Pseudorabies Virus dUTPase UL50 Induces Lysosomal Degradation of Type I Interferon Receptor 1 and Antagonizes the Alpha Interferon Response. Journal of virology, 91(21), e01148-17. [CrossRef]
- Zhang, R., Xu, A., Qin, C., Zhang, Q., Chen, S., Lang, Y., Wang, M., Li, C., Feng, W., Zhang, R., Jiang, Z., & Tang, J. (2017). Pseudorabies Virus dUTPase UL50 Induces Lysosomal Degradation of Type I Interferon Receptor 1 and Antagonizes the Alpha Interferon Response. Journal of virology, 91(21), e01148-17. [CrossRef]
- Zhang, S. Y., Boisson-Dupuis, S., Chapgier, A., Yang, K., Bustamante, J., Puel, A., Picard, C., Abel, L., Jouanguy, E., & Casanova, J. L. (2008). Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunological reviews, 226, 29–40. [CrossRef]
- Zhang, T., Liu, Y., Chen, Y., Wang, J., Feng, H., Wei, Q., Zhao, S., Yang, S., Ma, H., Liu, D., & Zhang, G. (2021). Antiviral activity of porcine interferon delta 8 against pseudorabies virus in vitro. International journal of biological macromolecules, 177, 10–18. [CrossRef]
- Zhang, X., Bai, X. C., & Chen, Z. J. (2020). Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity, 53(1), 43–53. [CrossRef]
- Zhang, X., Lu, S., Li, H., Wang, Y., Lu, Z., Liu, Z., Lai, Q., Ji, Y., Huang, X., Li, Y., Sun, J., Wu, Y., Xu, X., & Hou, J. (2020). Viral and Antibody Kinetics of COVID-19 Patients with Different Disease Severities in Acute and Convalescent Phases: A 6-Month Follow-Up Study. Virologica Sinica, 35(6), 820–829. [CrossRef]
- Zhang, Y. , Chen, S., Jin, Y., Ji, W., Zhang, W., & Duan, G. (2021). An Update on Innate Immune Responses during SARS-CoV-2 Infection. Viruses, 13(10), 2060. [CrossRef]
- Zhang, Y., Chen, S., Jin, Y., Ji, W., Zhang, W., & Duan, G. (2021). An Update on Innate Immune Responses during SARS-CoV-2 Infection. Viruses, 13(10), 2060. [CrossRef]
- Zhang, Y., Chen, Y., Li, Y., Huang, F., Luo, B., Yuan, Y., Xia, B., Ma, X., Yang, T., Yu, F., Liu, J., Liu, B., Song, Z., Chen, J., Yan, S., Wu, L., Pan, T., Zhang, X., Li, R., Huang, W., … Zhang, H. (2021). The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι. Proceedings of the National Academy of Sciences of the United States of America, 118(23), e2024202118. [CrossRef]
- Zhang, Y., Gargan, S., Roche, F. M., Frieman, M., & Stevenson, N. J. (2022). Inhibition of the IFN-α JAK/STAT Pathway by MERS-CoV and SARS-CoV-1 Proteins in Human Epithelial Cells. Viruses, 14(4), 667. [CrossRef]
- Zhao, J. Zhao, J., Zhu, F. C., Shu, Y. L., Zhou, R., Liu, L. Q., Zhang, L. L., Shi, Z. Y., Tang, Z., Lin, L. Z., Yu, Z. A., Zhang, L. P., Zhang, B., & Hou, Y. D. (2005). Zhonghua shi yan he lin chuang bing du xue za zhi = Zhonghua shiyan he linchuang bingduxue zazhi = Chinese journal of experimental and clinical virology.
- Zhao, J. Zhao, J., Zhu, F. C., Shu, Y. L., Zhou, R., Liu, L. Q., Zhang, L. L., Shi, Z. Y., Tang, Z., Lin, L. Z., Yu, Z. A., Zhang, L. P., Zhang, B., & Hou, Y. D. (2005). Zhonghua shi yan he lin chuang bing du xue za zhi = Zhonghua shiyan he linchuang bingduxue zazhi = Chinese journal of experimental and clinical virology.
- Zhao, X., Zhao, Y., Du, J., Gao, P., & Zhao, K. (2021). The Interplay Among HIV, LINE-1, and the Interferon Signaling System. Frontiers in immunology, 12, 732775. [CrossRef]
- Zheng, J., Mo, J., Zhu, T., Zhuo, W., Yi, Y., Hu, S., Yin, J., Zhang, W., Zhou, H., & Liu, Z. (2020). Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Molecular cancer, 19(1), 133. [CrossRef]
- Zheng, Y., An, H., Yao, M., Hou, J., Yu, Y., Feng, G., & Cao, X. (2010). Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. Journal of immunology (Baltimore, Md. : 1950), 184(11), 6447–6456. [CrossRef]
- Zheng, Y. , Deng, J., Han, L., Zhuang, M. W., Xu, Y., Zhang, J., Nan, M. L., Xiao, Y., Zhan, P., Liu, X., Gao, C., & Wang, P. H. (2022). SARS-CoV-2 NSP5 and N protein counteract the RIG-I signaling pathway by suppressing the formation of stress granules. Signal transduction and targeted therapy, 7(1), 22. [CrossRef]
- Zheng, Y. , Zhuang, M. W., Han, L., Zhang, J., Nan, M. L., Zhan, P., Kang, D., Liu, X., Gao, C., & Wang, P. H. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal transduction and targeted therapy, 5(1), 299. [CrossRef]
- Zhou, H., Zhu, J., Tu, J., Zou, W., Hu, Y., Yu, Z., Yin, W., Li, Y., Zhang, A., Wu, Y., Yu, Z., Chen, H., & Jin, M. (2010). Effect on virulence and pathogenicity of H5N1 influenza A virus through truncations of NS1 eIF4GI binding domain. The Journal of infectious diseases, 202(9), 1338–1346. [CrossRef]
- Zhou, Q., Chen, V., Shannon, C. P., Wei, X. S., Xiang, X., Wang, X., Wang, Z. H., Tebbutt, S. J., Kollmann, T. R., & Fish, E. N. (2020). Interferon-α2b Treatment for COVID-19. Frontiers in immunology, 11, 1061. [CrossRef]
- Zhou, Q., Chen, V., Shannon, C. P., Wei, X. S., Xiang, X., Wang, X., Wang, Z. H., Tebbutt, S. J., Kollmann, T. R., & Fish, E. N. (2020). Corrigendum: Interferon-α2b Treatment for COVID-19. Frontiers in immunology, 11, 615275. [CrossRef]
- Zhou, Q., MacArthur, M. R., He, X., Wei, X., Zarin, P., Hanna, B. S., Wang, Z. H., Xiang, X., & Fish, E. N. (2020). Interferon-α2b Treatment for COVID-19 Is Associated with Improvements in Lung Abnormalities. Viruses, 13(1), 44. [CrossRef]
- Zhou, R., Zheng, S. X., Tang, W., He, P. L., Li, X. Y., Yang, Y. F., Li, Y. C., Geng, J. G., & Zuo, J. P. (2006). Inhibition of inducible nitric-oxide synthase expression by (5R)-5-hydroxytriptolide in interferon-gamma- and bacterial lipopolysaccharide-stimulated macrophages. The Journal of pharmacology and experimental therapeutics, 316(1), 121–128. [CrossRef]
- Zhou, Y., Wang, M., Li, Y., Wang, P., Zhao, P., Yang, Z., Wang, S., Zhang, L., Li, Z., Jia, K., Zhong, C., Li, N., Yu, Y., & Hou, J. (2021). SARS-CoV-2 Spike protein enhances ACE2 expression via facilitating Interferon effects in bronchial epithelium. Immunology letters, 237, 33–41. [CrossRef]
- Zitvogel, L. , Galluzzi, L., Kepp, O., Smyth, M. J., & Kroemer, G. (2015). Type I interferons in anticancer immunity. Nature reviews. Immunology, 15(7), 405–414. [CrossRef]
- Znaidia, M., Demeret, C., van der Werf, S., & Komarova, A. V. (2022). Characterization of SARS-CoV-2 Evasion: Interferon Pathway and Therapeutic Options. Viruses, 14(6), 1247. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





