1. INTRODUCTION
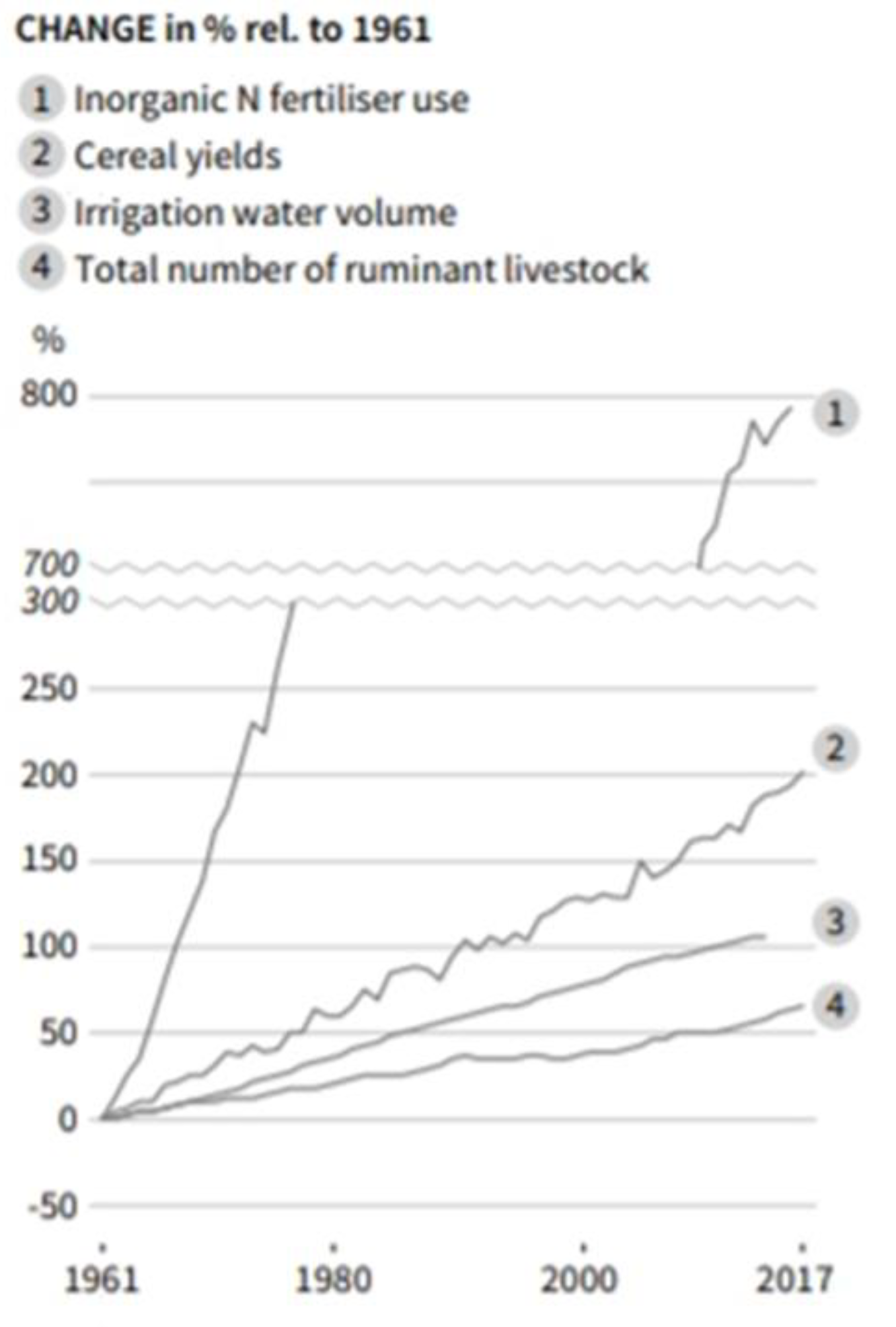
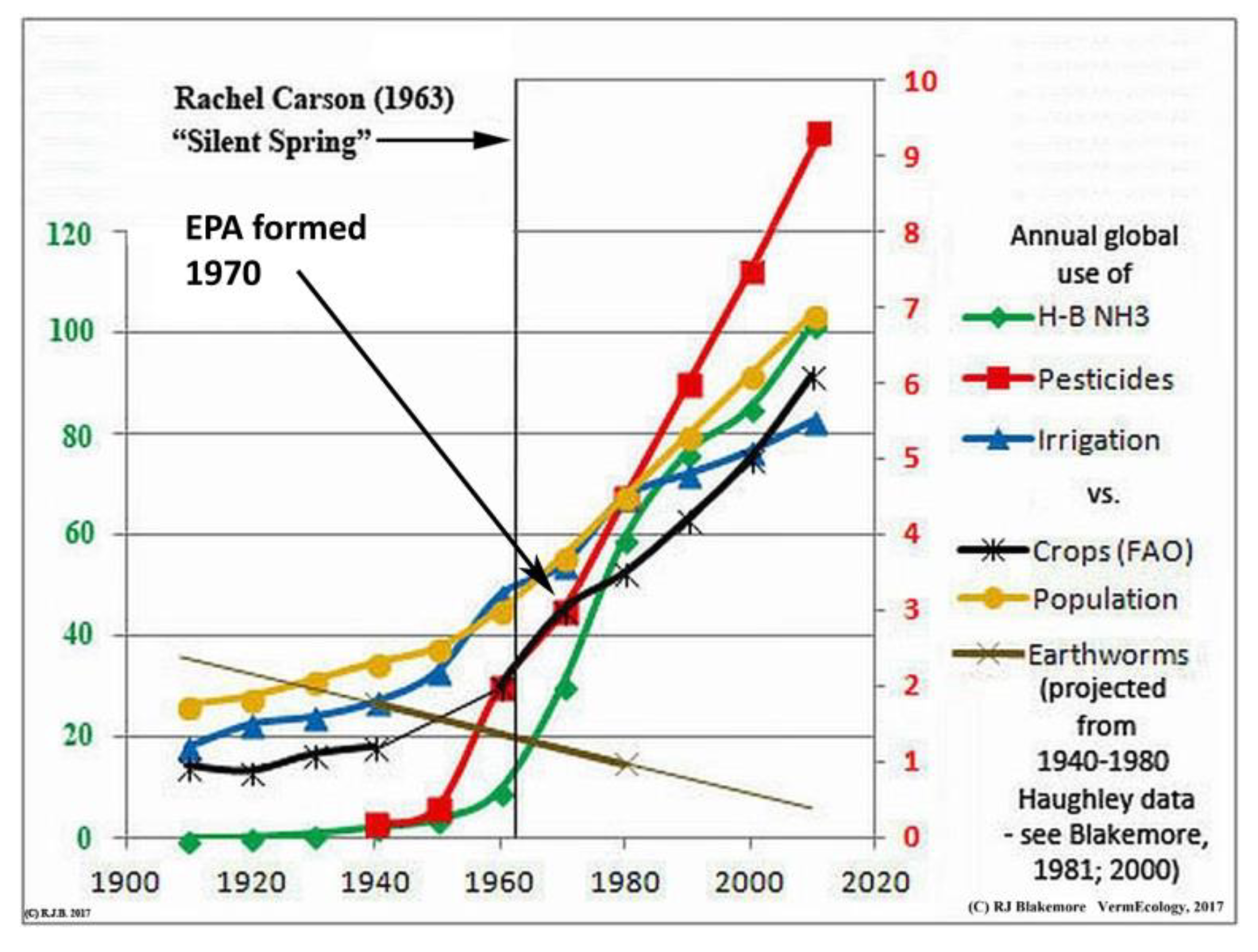
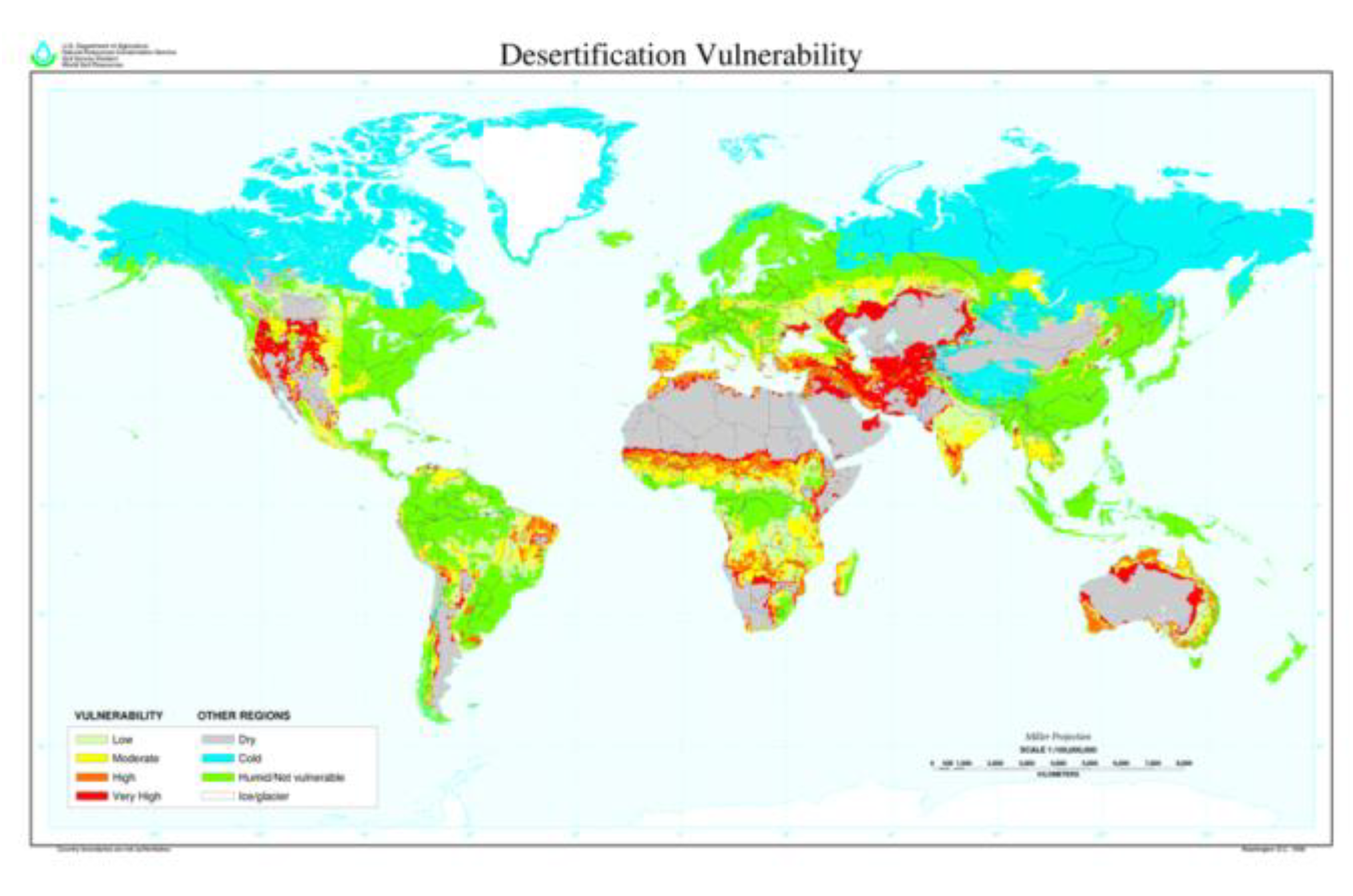
From Prehistoric times the expansion of agriculture and pastoral grazing have intensified forest clearance and topsoil erosion, now labeled Land-use Change (LUC). Consequences of erosion are soil organic carbon (SOC) loss to waterways or air with biotic declines ultimately leading to desertification. Realization of a growing soil deficit led to early works by organic pioneers such as Sir Albert Howard (1940, 1945) and Lady Eve Balfour (1943) that merit critical re-evaluation. Soil erosion and degradation, melting of boreal Permafrost, and peat loss are critical issues mostly downplayed or ignored in discussions of Ecological Sustainability or Climate (Koch et al. 2013, Guerra et al. 2020).
UN’s FAO (www.fao.org/3/i5199e/i5199e.pdf) reported: “Today, 33 percent of land is moderately to highly degraded due to the erosion, salinization, compaction, acidification and chemical pollution of soils.” Around the same time Gibbs et al. (2015) summarized: “FAO TerraSTAT interpretation of GLASOD by Bot et al. (2000), on the other hand, finds that over 6 billion ha [6 Gha], or 66 percent of the world’s land, has been affected by degradation, leaving roughly only a third of the world’s surface in good condition”. Information collated herein shows at least 50% depletion of arable soils following prehistoric agricultural expansion, with the loss rate having accelerated most rapidly under contemporary farm practices.
UK’s Dasgupta Review (2021) stated: “Collating data on soil erosion, WWF (2017) reported that some half of all top soils have eroded in the past 150 years. A typical estimate is that 75 billion tonnes of soil erode annually at a rate 13 to 40 times the background rates of erosion that prevailed before the acceleration caused by human dominance of the biosphere (Pimentel and Kounang, 1998). The rate of soil erosion accompanying land-use change is judged to be the highest in the past 500 million years (Wilkinson and McElroy, 2007), and some regard it to be the greatest geomorphic agent on the planet today (Hooke, 2000).”
The major environmental threat to Ecological Sustainability, productive capacity of agriculture, and species survival is soil degradation. Whereas a mountain stream – filtered by forest soils in carbon-balanced equilibrium – is crystal clear, agricultural areas mostly have brown rivers (also polluted) as well as dusty farm air. Rather than returning harvest “wastes” or manures to fields, these are variously disposed of or burnt and replaced by synthetic fertilizers, further depleting fertility inherent in topsoil humus. Farm soils, vulnerable if exposed, are mostly depauperate in biota and undarkened by carbon, a situation that requires fixing.
1.1. Why Are Soils So Important Yet So Poorly Known?
Nature and humanity depend upon soils for survival. Although being eroded (exploited?) at a massive scale, soils yet provide all our fibre/shelter materials, 99.7% human food, filter and store most of our freshwater supplies, gift many medicines (such as Penicillin, Streptomycin, Ivermectin, etc.), and support >99.9% of biodiversity and biomass although we know only a tiny fraction of the soil biota. As Dasgupta Review (2021) said: “The soils also supply most of the water needed by plants and for terrestrial biodiversity. Soil water makes up 65% of the world’s fresh water, is the source of 90% of global farm output and provides over 99% of our food calories”. This concurs with Pimentel & Burgess (2013 from “FAO, 2004” data): “humans worldwide obtain more than 99.7% of their food (calories) from the land and less than 0.3% from the oceans and aquatic ecosystems”. Despite paramount importance, global soil metrics remain devalued by unrealistic flat-Earth models with most focus and funding diverted – irrationally – towards Ocean, Aquatic, Air or Space research.
Soil ecological data are so poorly known that values may differ by an order or two of magnitude, often revised upwards. For example, global SOC stocks range 1,417–15,000 Gt C due to a statement by Hiederer & Kochy (2012) that: “the global SOC stock to 100cm soil depth is estimated at 1,417 Pg C” compared to best estimates (with terrain) of >10,000–15,000 Gt SOC (Blakemore 2018b, 2020c - veop.files.wordpress.com/2020/06/veop-4-5.pdf). Already (without terrain) errors were manifest in mineral soil underestimations up to seven times (Harper & Tibbett 2013), Permafrost by 200% or three times (Shelef et al. 2017), and total peat SOC was recently more than doubled (Loisel et al. 2021; Nichols & Peteet 2019, 2021). Roots are underestimated up to 100% (Robinson 2004), and Brovkin et al. (2011) found: “litter stocks based on observations (68–97 Gt C) or models (47–196 Gt C)” while for “terrestrial detritus” Reiners (1973) had “over ten times Bolin‘s estimate”. Mainly terrestrial Bacteria have uncertainty, as with most other soil biota, up to 10-fold (Bar-On et al. 2018). For Net Primary Productivity (NPP), estimates were 2–5 times higher accounting for belowground dynamics (Scurlock et al. 2002), and Running et al. (2004) discuss disparities in both satellite and model assumptions with: “range of two orders of magnitude in field-measured NPP”. Soil erosion has rate of loss: “unsustainable at 10–1000 times higher than the rate at which soils form” (Kopittke et al. 2019). Winkler et al. (2021b) posited global land use change as 4 x greater than previously estimated. Koren et al. (2019) show soil<->air CO2 flux varies from 25–450 Gt C/year, and the discrepancy of SOC loss oxidation fraction during erosion ranges 0–100% (Lal 2006: tab. 3.3). Preindustrial SOC loss estimates differ by 48–540 Gt C (van Oost et al. 2012, Kaplan et al. 2010: tab. 3) while Postindustrial data also vary with conversion from natural ecosystems to farmland in ~170 years post-1850 causing SOC losses to the atmosphere of between 50–200 Gt C (e.g. Lal 2006, 2009; Appendix A).
Despite recent initiatives such as GBIF, SoilBON, Global Fungi Database (https://globalfungi.com/) or Earth Microbiome Project (https://earthmicrobiome.org/), data deficits in soil information relate to lack of a dedicated Soil Ecology Institute to compile or coordinate basic research/education, an issue that is further discussed below.
1.2. True Topographic Land Surface Area Recalibrations
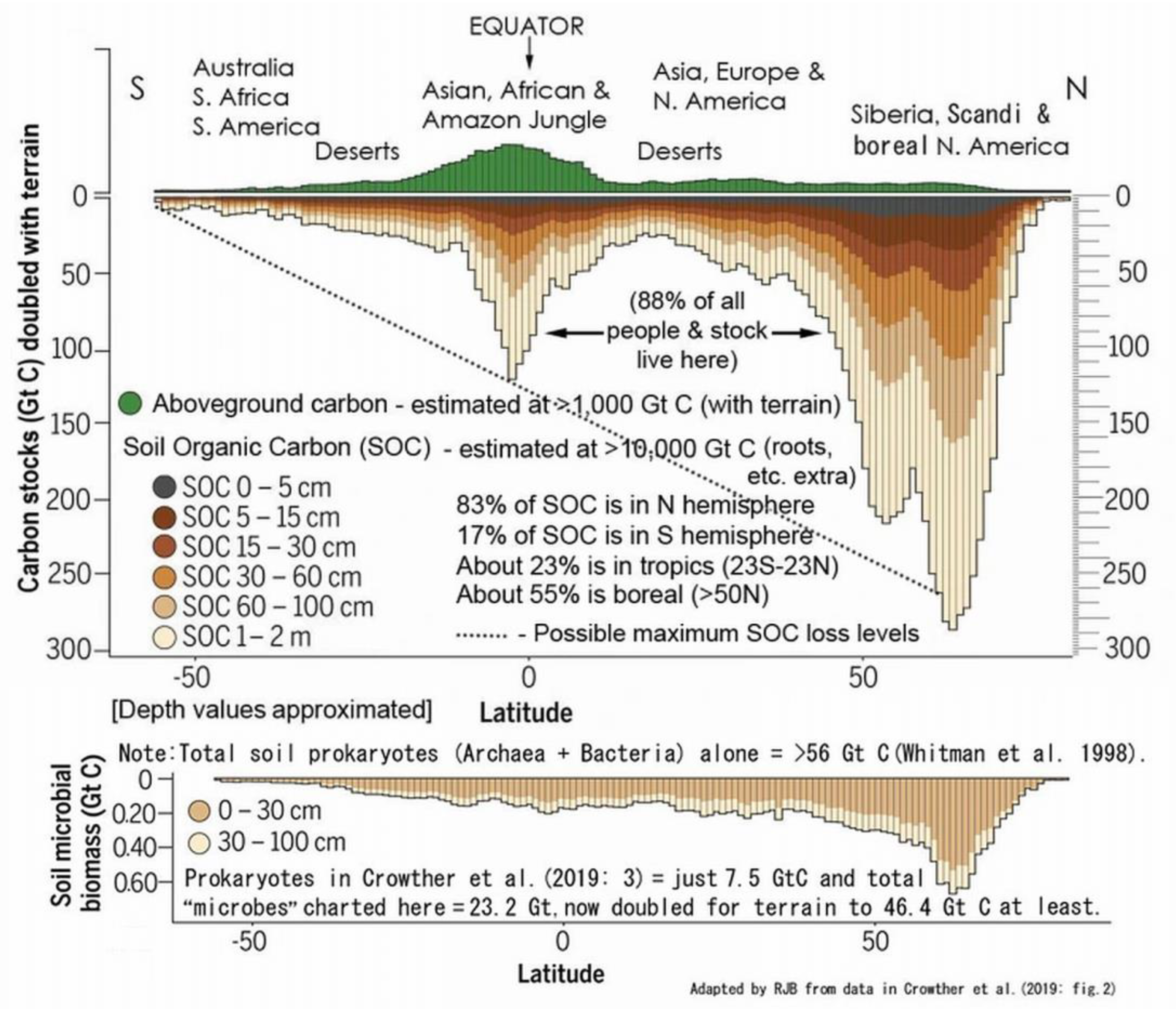
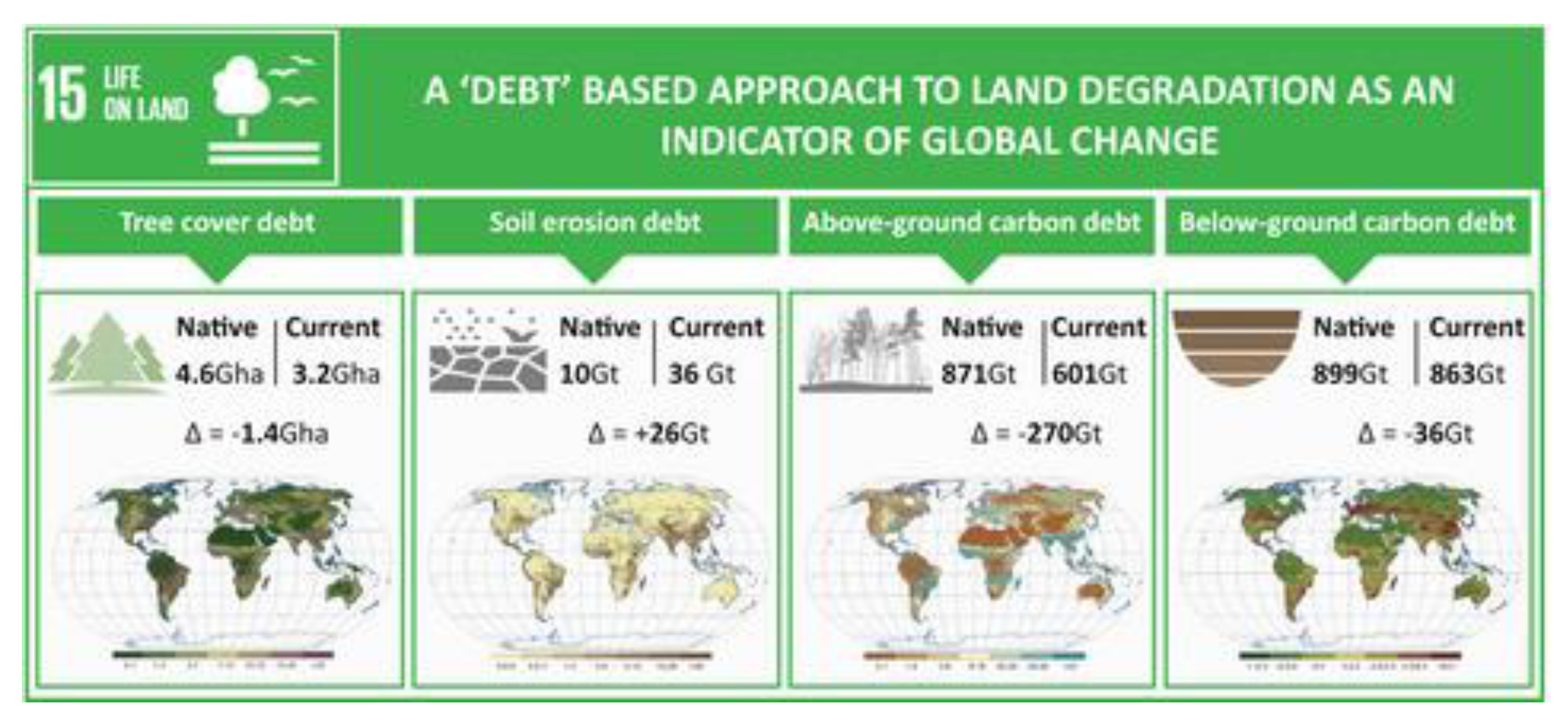
Most calculations of global metrics assume an unrealistic, planimetrical flat-Earth terrain of just 15 Gha, ignoring the manifest fact that land is hilly and soil is bumpy. Neither NASA nor NOAA provide a global, terrestrial topographical surface-area total, thus it is beholden upon individual researchers to compile data. A summary by Blakemore (2018b) effectively doubled land area at coarse metre scale and quadrupled it with overlain or superimposed finer cm and mm scales. Prior to this – on the false premise that land topography and soil rugosity are irrelevant compared to the large size of the planet – calculations assumed a mirror-flat Earth. Whereas terrain increases actual land values to >30–64 Gha, relative proportions remain roughly the same except for aquatics (e.g. mangroves 0.1% or inland water bodies around 1%, both at least halved) (
Figure 1).
Recalibration allowance for Earth’s topography was updated by Blakemore (2019a, b, 2020a, c), one consequence was substantial increase in SOC totals. Earlier estimates by 4p1000.org from Hiederer & Kochy (2012) having “Global SOC Stock Pg 1,417” as quoted by FAO (e.g. www.fao.org/3/i7268e/i7268e.pdf; www.fao.org/3/i6937e/i6937e.pdf), or of ~5,000 Gt C by IPCC or SoilGrids, were raised up to 10,000–15,000 Gt C for terrain, etc. Higher SOC values were seemingly acceptable to Dr Rattan Lal (2019a, 2019b, 2020).
Blakemore (2018b) clarified that: “Tangible sub-samples are taken on the ground at fixed core sample volumes with a constant planimetric area (cm−2 or m−2 perpendicular to the centre of the Earth) and then multiplied by a biome’s area.” Thus when biome area increases so too do associated values. Recent survey methods include flux towers or satellites and whereas most ice-core, water, or atmospheric data (and some soil samples?) are reliably immutable, all flat biome extrapolations that neglect terrain factors almost certainly underestimate totals. Greater soil surface naturally accommodates more plants and leaf exposure to basic elements thereby validating increases in biotic SOC and NPP values.
Allowances for terrain increase most soil and biota data, but changes wrought (as herein) are cardinal rather than exponential. Upping land surface from planimetric 15 Gha to topographic >30–64 Gha at least doubles NPP, total litter, earthworm biomass, etc. Abundance counts too, for example ubiquitous ants jump from 20 × 1015 to 40 × 1015 ants.
1.3. Prehistoric and Historic Rates of Agricultural SOC Losses
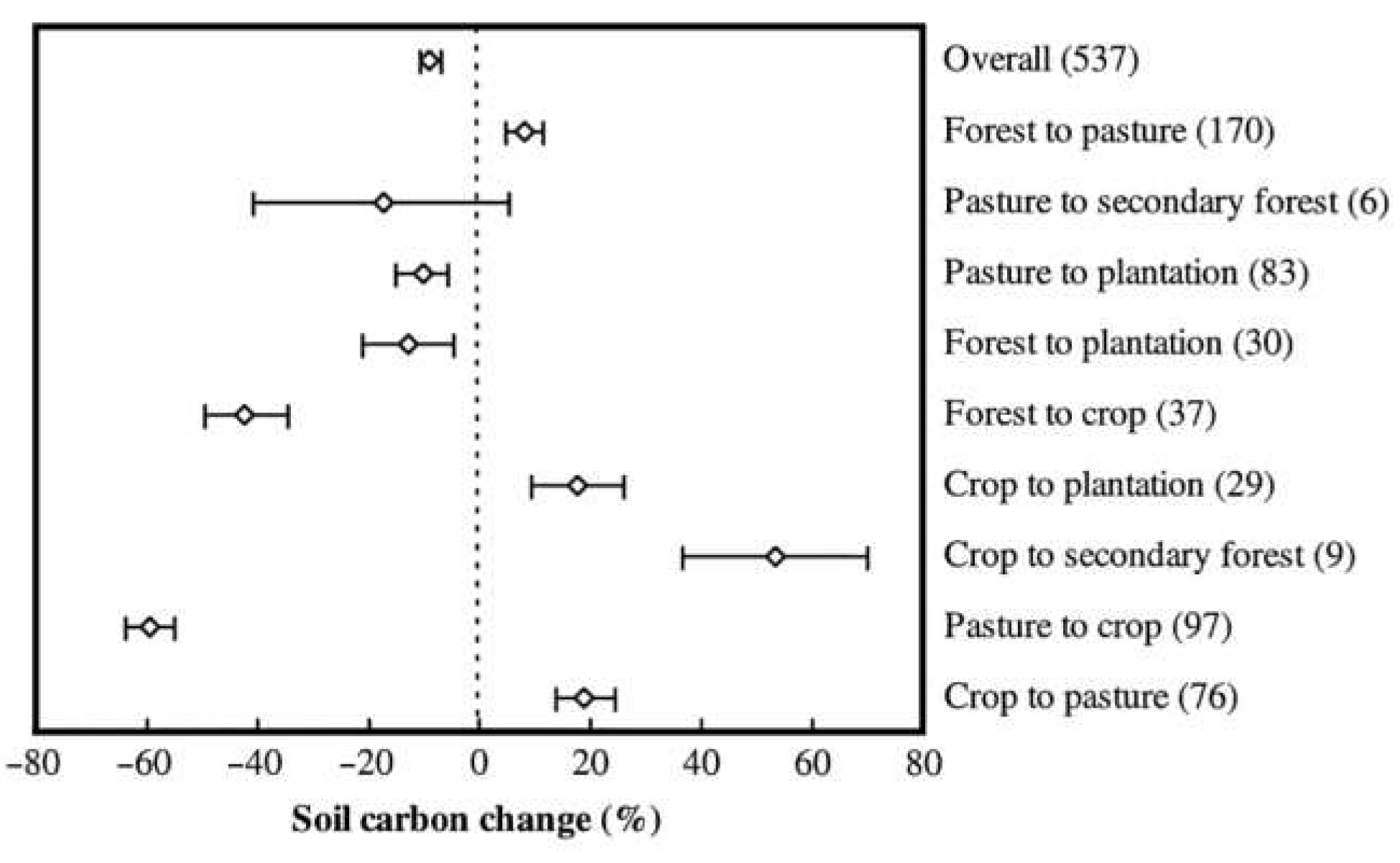
Land or vegetation shifts are from natural Ecological Succession or are due to human activities. Since prehistoric times, humans have unwittingly caused a critical loss of organic matter from soil above natural recycling levels due to “fire-stick farming” and clearing of forests for grassland or crops (Ruddiman 2003, Ruddiman et al. 2016). Organic carbon derived from living organisms is stored naturally in soils to depth; Buringh (1984) estimated 537 Gt SOC loss from an initial 2,014 Gt (“prior to the spread of civilizations in the last two millennia”) down to 1,477 Gt (i.e., 60 Gt more than Hiederer’s 1,417 Gt although neither guesstimate considers deeper soils nor Permafrost cryosols). Buringh (1984: tab. 3.5) had average soil carbon in forest to cropland conversion depleting (208 - 95 =) 113 t/ha but forest to grassland loss slightly less at 92 t/ha. As percentages, these approximate -54 to -44%, respectively (a mean loss from all forest soil of around -50%).
This halving of SOC stocks could presumably be reversed via forest restoration. A meta-analysis by Guo & Gifford (2002: fig. 1) shows both positive and negative SOC responses to various LUC events covering 16 countries but mainly: Australia, Brazil, NZ, and USA. However, as human population since 1980s doubled from 4 to 8 billion today, decisions to clear forest for pasture/cropland requires informed and balanced viewpoints.
Elhacham et al. (2020) also claim humanity has roughly halved the mass of plants since the Neolithic agricultural revolution, from about 2,000 Gt dry-mass down to the current value they say of ~1,000 Gt; or from about 1,000 to 500 Gt C. This carbon has presumably been redistributed elsewhere (to soil, to air, or to waterways). Furthermore, in the last 8,000 years, conversion of natural vegetation for agriculture has resulted in detrimental erosion of around 27,187 (± 9,030) Gt of dry topsoil worldwide according to Wang & Van Oost (2019). This they said resulted in an average cumulative sediment mobilization of 1,829 (± 613) kg m−2 for croplands corresponding to a mean soil truncation (or loss of depth) of ca. 1.34 (± 0.45) m. These authors estimate a similar depth of cropland soil (“a mean of ca. 1.3 m”) remains globally, implying 50% depth of crop soil is gone.
Taking SOC as 2.5%, which may be reasonable for upper layers of topsoil, gives displacement of (27,187 x 2.5% =) ~680 Gt C, or about twice that calculated by IPCC (2013: 484): “The δ13C of atmospheric CO2 trapped in ice cores can be used to infer changes in terrestrial biospheric carbon pools”.. “a modelling study by Kaplan et al. (2011) suggested that more than 350 PgC [350 Gt C] could have been released as a result of LULCC [land use/land cover change] between 8 ka and 1850”. Kaplan et al. (2010) concluded: “Anthropogenic deforestation, followed by soil erosion, and land degradation, may have even resulted in irreversible ecological shifts.”
Erosion since 1850, if at 75 Gt/yr (Myers 1993, Pimentel et al. 1995, Pimentel & Burgess 2013: 447), sums to ~13,000 Gt dry soil (x 2.5% = 325 Gt SOC) accounting for near half topsoil loss due to agriculture over time (viz., 350 pre-1850 + 325 post-1850 = 675 Gt SOC).
Compared to this current estimate of around 675–680 Gt SOC loss, prior cumulative soil carbon losses and emissions over the Holocene (or in the last ~10,000 years) as a result of anthropogenic land and soil erosion had been estimated at 456–537 Gt SOC (see Appendix A). Most studies however, omit a likely considerable loss of vegetation and soil prehistorically and presently occurring on the Australian continent. This will to be discussed in further detail later, but of note is that Lal (2006: tab. 3.5) has lowest SOC erosion and C emissions from “Oceania” at about half those from Europe albeit both continents are similar in size, each being roughly ~6% of total land area.
As well as a soil sustainability imperative, SOC loss is a key contributor to CO2 increase. Three interlinking factors are actual SOC loss, Land Use Change (LUC), and fires.
1.4. Recent Rates of SOC Loss, LUC, and Fire Contributions to Atmospheric Carbon
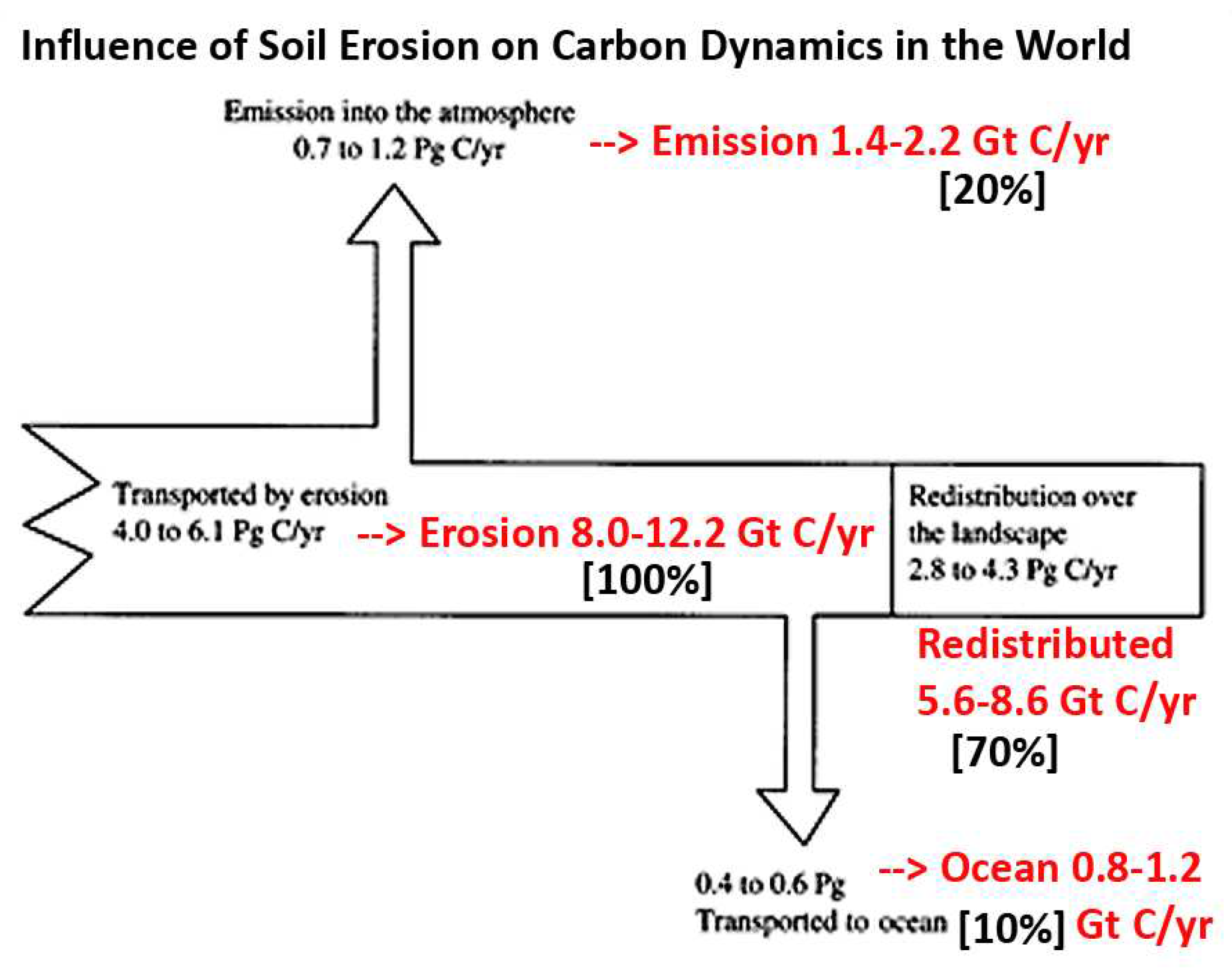
Soil erosion rates due to human activities have accelerated in recent times but estimates differ widely. Buringh (
1984: tab. 3.8) had total excess SOC released to the atmosphere, mainly from clearing of forests (including soil C loss from fuel wood and forest fires), ranging 2.5–7.4 Gt C/yr with 4.6 Gt C/yr he considered a realistic mean value. Comparatively, Lal (2004: tab. 1) had 136 Gt C/yr LUC loss to the atmosphere in 150 years, or a rate of ~1 Gt C/yr. Later, Lal (2006: fig. 3.2, tabs. 3.4–3.5) had SOC loss as ~5 Gt C/yr with most (~70%) redistributed over the landscape; ~20% lost to the atmosphere, with the remaining ~10% flowing to the ocean via rivers. Along with massive coastal erosion, Ocean carbon budgets may thus largely relate to changing rates of soil transportation, as is shown in graphical review data (
Figure 2).
Regarding SOC erosion contribution to atmospheric carbon: Early on, Hutchinson (1954) had suggested terrestrial biota (especially soil microbes?) were a net source of CO2 for the atmosphere, gaining some ideas from V.I. Vernadsky’s concepts of the Biosphere.
More than 45 years ago, Schnitzer & Khan (1978) had shown the decay of organic soil matter provides the largest carbon dioxide input into the atmosphere, more so than fossil fuel emissions. Woodwell et al. (1978) agreed saying: “analysis shows through convergent lines of evidence that the biota is not a sink and may be a source of CO2 as large as or larger than the fossil fuel source.” They continued: “Because of the paucity and uncertainty of data the actual rate of release is elusive; under extreme conditions it could be as high as 18 x 1015 grams of carbon per year [18 Gt C], or more than three times the annual release of carbon through combustion of fossil fuels, currently estimated as about 5 x 1015 g ” [5 Gt C]. Lal & Pimentel (2008) also argued that: “soil erosion is a strong source rather than sink of atmospheric CO2”.
This is consistent with an earlier IPCC (2000) report of “a global net terrestrial source” to excess CO2 rather than a sink. As Lal (2001: 533) stated (bolding added): “The atmospheric C pool is increasing at the rate of 3.3 PgCyr-¹ [now 4.7 Gt C/yr] primarily at the expense of the soil and the biotic pools [on land]”. Using depletion of C or O isotopes as evidence that atmospheric CO2 is mainly from fossil fuel sources is negated by soil’s antiquity.
Nevertheless, IPCC (2001 - www.ipcc.ch/site/assets/uploads/2018/02/TAR-03.pdf) in its ~400 page report yet claimed: “Several additional lines of evidence confirm that the recent and continuing increase of atmospheric CO2 content is caused by anthropogenic CO2 emissions – most importantly fossil fuel burning. First, atmospheric O2 is declining at a rate comparable with fossil fuel emissions of CO2 (combustion consumes O2). Second, the characteristic isotopic signatures of fossil fuel (its lack of 14C, and depleted content of 13C) leave their mark in the atmosphere. Third, the increase in observed CO2 concentration has been faster in the northern hemisphere, where most fossil fuel burning occurs.” All three claims (repeated almost verbatim in another ~800 page IPCC report by Canadell et al. 2021: 689 - www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Chapter05.pdf), are appropriately attributable as much, if not more so, to the complexities of LUC and net SOC loss than simple fossil fuel (FF) burning, as is demonstrably proven (Q.E.D.) herein.
Yet uncertainties remain large, e.g. Li et al. (2014) found an extra 50% SOC erosion of black-soil chernozem/mollisols in China that may reduce fertility of crops 40% (Delang 2018) emphasizing an imperative to restore SOC for sustenance as well as for climate.
Despite such information, Ussiri & Lal (2017) mistakenly stated: “At the beginning of Industrial Revolution, the emissions of CO2 were from land use and land use change; now the emissions are largely (~90%) from fossil fuels” whereas, factually, the reverse is true. Reviews by Oertel et al. (2016) and Gerke (2022) show what Hayes & Clapp (2001) stated two decades ago is more realistic, namely, worldwide net release of CO2 from soils is higher by a factor >10 x than CO2 released from fossil fuels. Thus a false idea is promoted that FFs are most problematic rather than it being more of a soil issue. Data presented herein tend to support this conclusion with soil respiration (SR) at 10–20 x (or ~90–95%) FF emissions as detailed below. Urgency of reversing SOC loss to erosion or degradation – regardless of its major contribution to increase of atmospheric CO2 content – is also emphasized in a recent “GLO2” Report (UNCCD 2022) on the threats of desertification.
The current study reviews interlinked LUC and SOC loss contributions to increasing atmospheric CO2 showing these more important than FFs. Furthermore, a failure of photosynthesis to fix excess CO2 is due mostly to soil erosion and vegetation degradation. Simply halting FFs does nothing to drawdown CO2 carbon. SOC restoration alone does this whilst reversing desertification and provisioning healthy food productivity.
1.5. Net Primary Productivity (NPP) Builds SOC vs. Soil Respiration (SR) Depletes
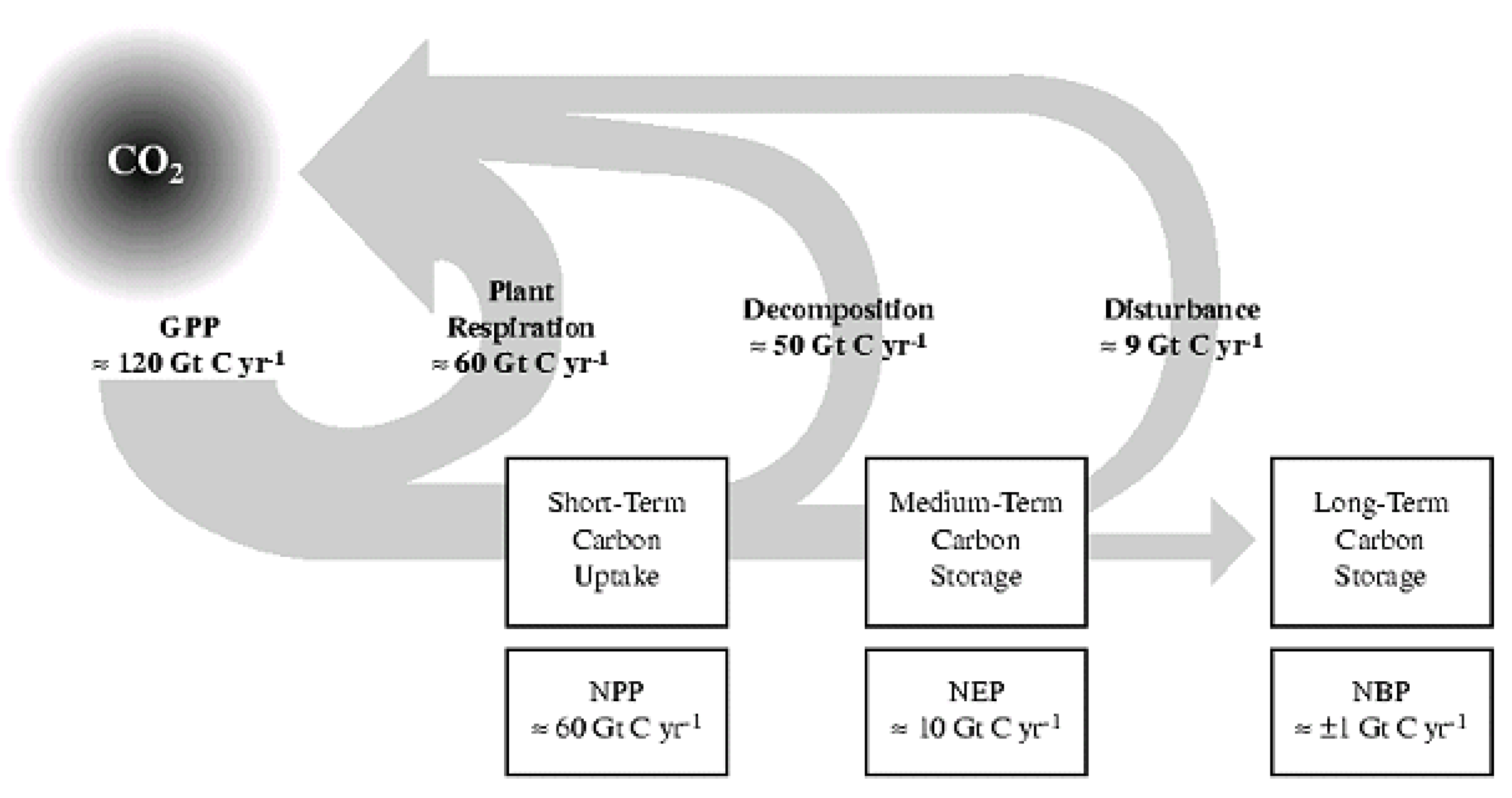
Annual budgets of carbon naturally respired or decayed from soils (SR) is many times the erosion values given above. As already well known, terrestrial GPP/NPP is the most significant carbon cycle on Earth. ESDD (2022: fig. 2) now shows FF +9.5 Gt C/yr (big arrow) vs. GPP terrestrial exchange of ±130 Gt C/yr (small arrows). With a similar amount of respiration, presumably half (~65 Gt?) from soil, gives NPP ~65 Gt C/yr. These are obvious underestimations as net soil CO2 emissions in Bahn et al. (2010) are ~98 Gt CO2 C/yr, while Oertel et al. (2016) and Gerke (2022) have annual rate of 350 Gt CO2e (= ~94.5 Gt C?), both 10 x FF of ~9 Gt C/yr. Thus soil respiration, “officially”at ~98 Gt CO2 C/yr, when reasonably doubled for neglected terrain to ~196 Gt C/yr, gives soil respiration (SR) ~20 x fossil fuels (FF) (cf. Blakemore 2020c).
Soil Respiration at ~100–200 Gt C/yr implies ~100–200 Gt C/yr Plant Respiration (PR) to total ~200–400 Gt C/yr NPP balancing the formula of NPP = GPP – (PR + SR) (
Figure 3).
If terrain doubles Fig. 3 “Disturbance” to 18 Gt C/yr (cf. HANPP noted below of 15 Gt C/yr) and if just half is due to human LUC, then this 9 Gt C/yr equals a soil erosion rate from Buringh (1984) (4.6 x 2 = 9.2 Gt C/yr) and also equates to ~9 Gt C/yr FF emissions.
Rather than accepting LUC matches FF emissions, the latest report (ESSD 2022: fig. SPM1) shows net LUC (including soil) just 11% of total. Hence, as the current report demonstrates, calculations ignoring terrain may underestimate LUC by a factor of at least two. And while the “official” claim of NASA/NOAA/CSIRO/IPCC is of terrestrial NPP and SR both at 60 Gt C/yr (86%) with FFs emitting ~10 Gt C/yr (ca. 14%), many other reports provide higher estimates. For example, isotope studies by Welp, Keeling et al. (2011) state: “Our analysis suggests that current estimates of global gross primary production, of 120 petagrams of carbon per year, may be too low, and that a best guess of 150–175 petagrams of carbon per year better reflects the observed rapid cycling of CO2”, i.e., giving approximate NPP, PR, and SR rates each of >80 Gt C/yr with FF contribution then only ~11%. Rodin et al. (1975) already had Land’s NPP of 86 Gt C/yr that, when increased for greening effect, is now over one hundred Gt C/yr (and doubled for terrain is >200 Gt C/yr). Other “official” counts are also of higher NPP, e.g. IPBES (2018: 347) has “a total global terrestrial NPP of around 100 PgC yr-1” trending towards an estimate, with terrain, of 218 Gt C/yr (Blakemore 2018b), that then reduces FF contribution to just 4–5% of terrestrial C output. This ignorance of terrain is perhaps a main reason why many models fail to adequately represent reality nor to actually help to remedy problems of the global carbon cycles.
1.6. Context of Soil in Global Carbon Budget
By mass, Carbon (C) is the fourth most abundant element in the Universe after hydrogen, helium, and oxygen (O). On Earth’s crust, carbon as the 15
th most abundant element is transformed into a variety of organic compounds, comprising about 50% of dry biomass. UNEP’s (
2002: tab. 2.1) “
World Atlas of Biodiversity” total carbon content is newly revised to about 81,060,000 Gt (
Table 1). This clearly shows Soil has by far Earth’s greatest active store of carbon, much greater than the Ocean’s despite contradictory claims. It is also pyramidion for plant growth (vermecology.files.wordpress.com/2017/10/npk.jpg).
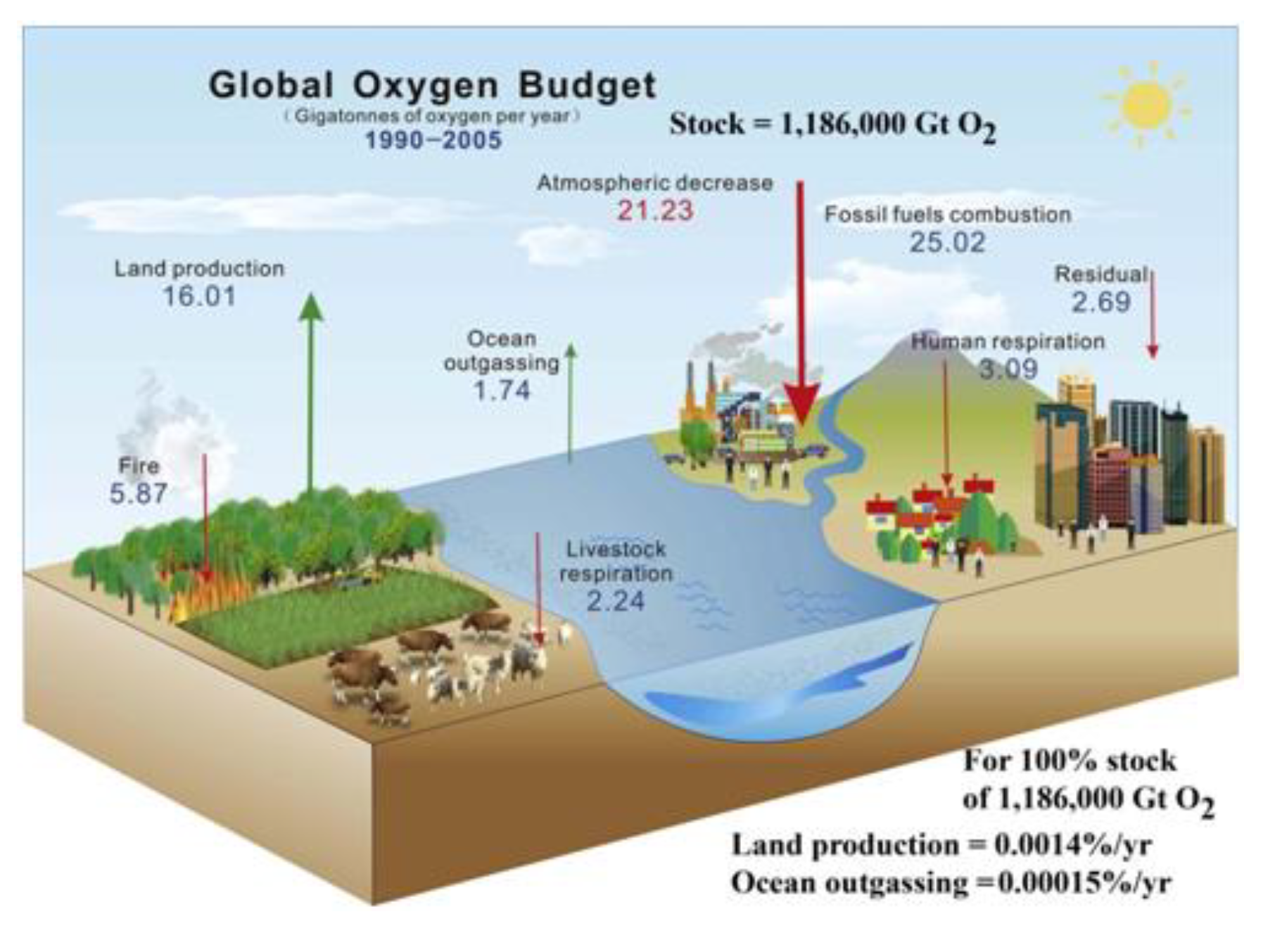
As well as SOC, complete soil carbon stock inventory broadly includes root/mycorrhizae, logs/leaf-litter, biocrusts, and various other soil biota that add considerably, as is discussed below. Current terrestrial carbon of at least 2,000 Gt in above- and below-ground plants plus many soil organisms mostly intermixed with the 10,000–15,000 Gt SOC in humus as active carbon stored and recycled on land. This compares with latest ESSD (2022: fig. 2) values of just 450 Gt C in Vegetation, 1,400 in Permafrost and 1,700 in Soils (total 3,100 Gt C) (cf. just 3 Gt C in Marine biota and 700 Gt C in Marine Organic carbon to total 703 Gt C total Ocean organic carbon). Although ESSD now accepts an active terrestrial GPP of 130 Gt C/yr (= ~65 GtC/yr NPP?) versus alleged, mostly passive, 80 GtC/yr ocean<->air exchange, a higher active GPP rate on land of ~440 Gt C/yr, as is justified herein, naturally recycles all atmospheric CO2 in around a two year turnover time.
1.7. Context of Biotic Abundance, Biodiversity and Biomass
Most biodiversity on Earth is microbial (Prokaryota mainly Bacteria, Archaea, etc.) with immense numbers compared to Eukaryota that numbers only in millions or billions of taxa. (The question whether endosymbiotic mitochondria found inside nearly all eukaryote cells – as used for mtDNA barcode identification – also represent separate species is outside the scope of the present work). For biomass, a much-cited study by Whitman et al. (1998: tab. 5) estimated total prokaryote cellular biomass at 353–546 Gt C (median ~450 Gt) they claimed almost equaled carbon storage in phytomass of land plants (~560 Gt C). Allocation of prokaryotic cells was 3.67 x 10
30 in oceans or 2.76 x 10
30 on land (with 305 Gt C and 241 Gt C, respectively). These totals were revised by subsequent researchers, not least Bar-On et al. (2018) who had just 77 Gt C microbe biomass. Microbe biodiversity was reviewed by Louca et al. (2019), (with abundance) by Locey & Lennon (2016) and by Zhao et al. (2022) as summarized below for all six Realms-of-Life (
Figure 4,
Table 2).
Deep carbon data are of less practical concern to the current study on soils and land’s carbon cycles, although they again highlight deficiency of Ocean’s excessively claimed biota at all scales and at all depths. Deep subsurface biota were briefly reviewed by Blakemore (2022); earlier, McMahon & Parnell (2013: tab. 3) refined Whitman et al.‘s (1998: tab; 5) estimated continental subsurface biomass to 14–135 Gt C in the top 2 km of continental crust. Bar-On et al. (2018) update was soon revised by Magnabosco et al. (2018) for continental subsurface biosphere to 2–6 × 1029 cells, <105 taxa, and 23–31 Gt C. Moreover, Whitman et al.’s Ocean subsurface sediment of 303 Gt C was reduced by Kallmeyer et al. (2012) to just 4.1 Gt C. For ocean deep subsurfaces, Bar-On et al. (2018) appear to have confirmed some approximate ratios, while Hoshino et al. (2020) provided an update of 1028–1029 cells, 103–106 taxa, and just 35 Gt C.
Flat-Earth areas from Whitman et al. (1998: tab. 2) total 12.3 Gha that they multiplied by numbers of microbe cells sampled from each biome. Terrain at least doubles the land count and thus their total biomass. Thus land’s allocation is greatly increased proportional to that of the Ocean for Earth’s total biota. Albeit taxa migrate variously between the six main Realms-of-Life, as shown by Zhao et al. (2022), the main habitat distinction maybe defined according to Grosberg et al. (2012) along the lines of where species breed or spend the majority of time (living, dormant, or deceased?). Condensing to Super-realms, we may broadly refer to those species that breed in or on soils (the “Soliota”?) to the relatively fewer mainly aquatic species (“Aquaota”?). [Ronin et al., 1975 use a term “Aquatoria” for aquatic biota but this term has other meanings].
Great biotic superiority in abundance, diversity and biomass of Soil compared to the other five Realms-of-Life (Tab. 2) is accounted for by its relative stability and lack of mixing by winds or currents. Each soil is unique with its biota evolving endemically. Thus, resident earthworms abide, speciating in situ, their global distributions helping explain Wegener’s Plate Tectonics. In contrast, alate ants or termites, as for other winged insects or spiders, disperse widely. Proper context readily allows triage of most urgent or important key-issues in order to try to preserve all biodiversity (including humanity).
1.8. Aims of the Current Study
The current study is on topographic terrain, biotic SOC status, and organic Permaculture solutions to erosion and extinctions. To clarify, as noted above, it is already well established that soil organic matter emissions exceed those of fossil fuels and that erosion greatly exceeds natural soil formation. The current study re-evaluates current estimates of biotic carbon and balanced cycles in context with triage, highlighting urgency of key issues while suggesting practical remedies. Important concerns of global species extinction, soil erosion, human sustenance and farming sustainability are critically reviewed.
Three important questions are: What are true soil organic carbon (SOC) stocks and NPP rates (and what are above- or below-ground biomass/necromass allocations)? How much do estimated rates of historical or current soil erosion contribute to accelerating CO2? Can agricultural SOC loss be realistically reclaimed via 100% organic restoration?
3. RESULTS & DISCUSSION
PART 1 – SOC Stocks
SOC tallies are obtained from samples, taken perpendicular to the centre of the Earth typically in fixed volume cores (for bulk density) with dried soil mm-sieved. Stones (rare in organic or worm-worked soils), roots, or megafauna (such as earthworms) are removed. Surface litter, often treated separately, is essentially included; roots rarely are. Sample data is then extrapolated for areas. Biomass of microbes (living, dead, or dormant) is integral making up as much as 80% of humic SOC (Gross & Harrison 2019).
Superficial soil samples (to 0.3–1 m) greatly undervalue SOC: Jobbagy & Jackson (2000) reported 56% more SOC at 3 m compared to 1 m, and D’Elia et al. (2017) found “SOC in soils quantified from 0–3 m were on average more than double the SOC from 0–1 m“. For mineral soils, Harper & Tibbett (2013) further found two to five times greater SOC (average ~3.5 times) at depth to bedrock of >5–38 m, with “major implications for estimates of global carbon storage and modelling of the potential global impacts of climate change and land-use change on carbon cycles”. Permafrosts may extend to 1.5 km depth and peats are recorded up to ~200 m; notwithstanding Permafrost SOC estimates may increase >200% (Shelef et al. 2017) and peat SOC was expanded (Loisel et al. 2021; Nichols & Peteet 2019, 2021).
Where soil-bearing land is the main consideration, Earth’s total flat, ice-free area of 10–12 Gha is doubled (× 2) for terrain to 20–24 Gha as a justification for current revisions.
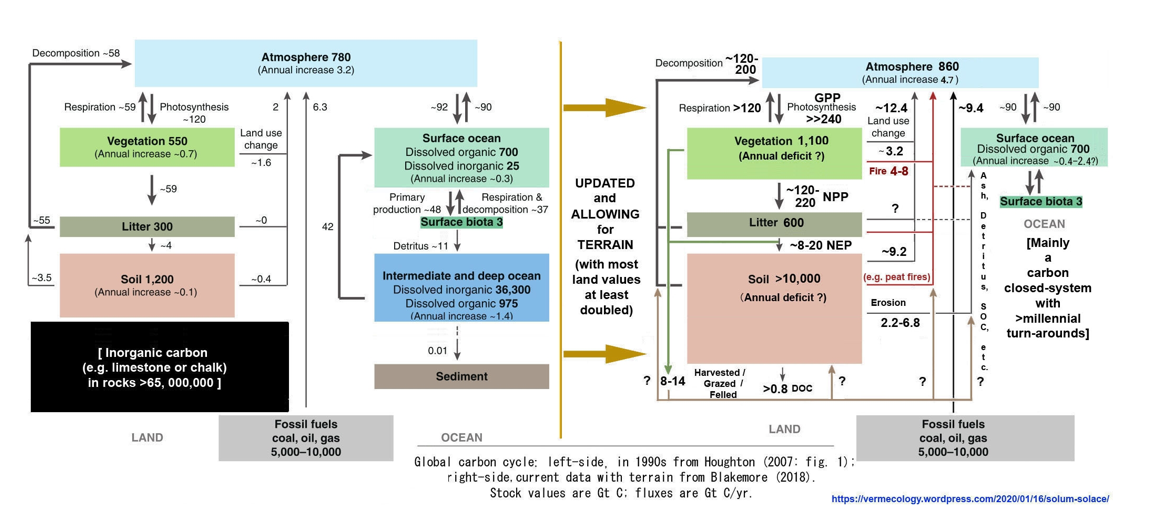
3.1. Reconciling Current SOC Stock and the Global Carbon Cycle
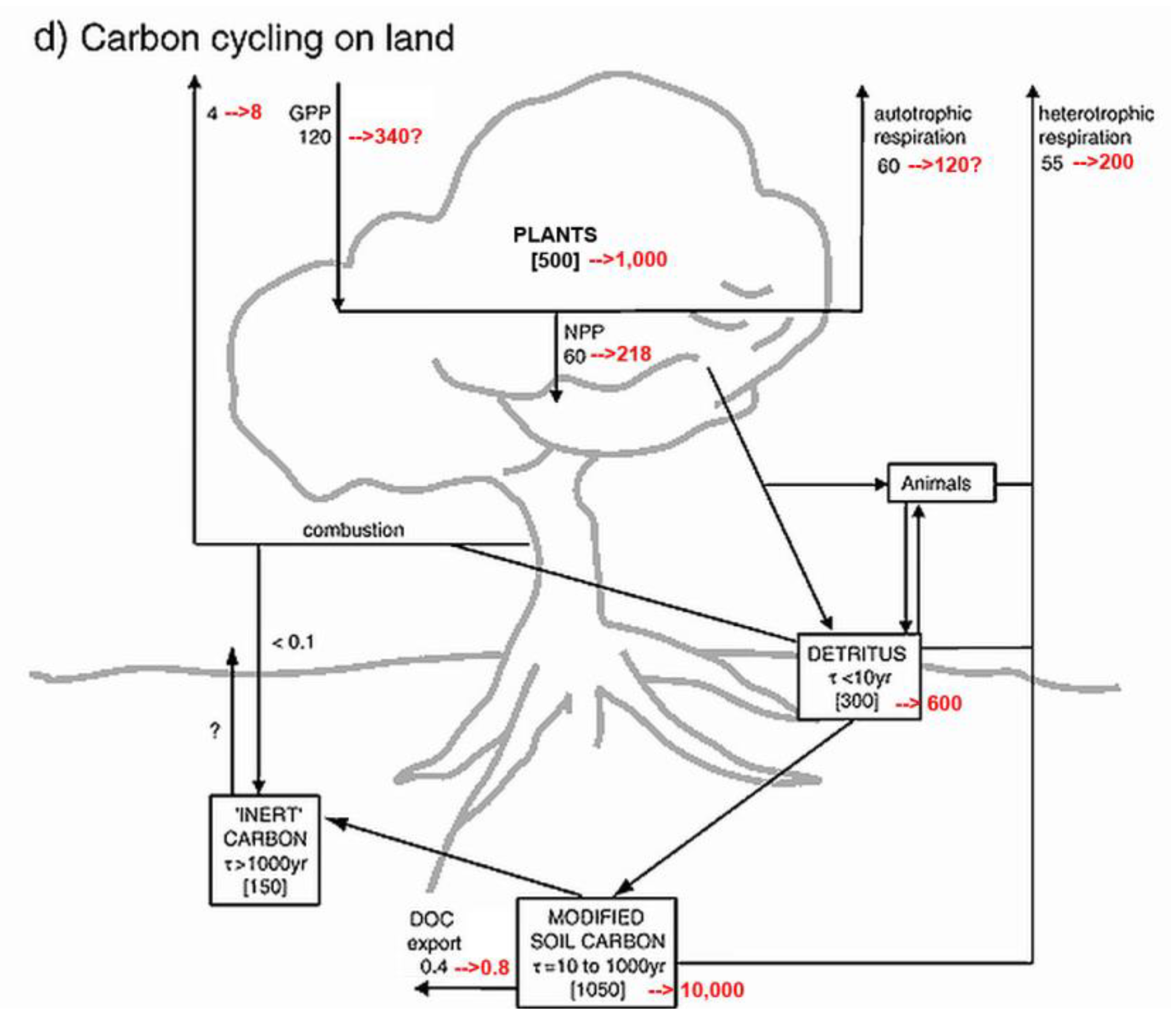
Soil offers hope and solace: The present report demonstrates that topsoil is
the major source of annual carbon emissions and Earth’s greatest carbon storage with at least 10,000 Gt total C in soil organic carbon (SOC) alone. A starting point for review of SOC total is IPCC (
2001: fig. 3.1 WG1-TAR1) with 1,200 Gt plus 300 Gt plant litter “
detritus” that were almost immediately contradicted by their table 3.2 data showing SOC between 1,567–2,011 Gt to 1 m depth and a statement that peatlands contain an extra 455 Gt SOC, both easily doubled for greater depth to give 4,000–5,000 Gt, most now also doubled for terrain to total 8,000–10,000 Gt SOC (Blakemore
2018b, 2019b, 2020a). IPCC (
2001: tab. 3.2 WG1-TAR1) shows that their calculations were based upon a flat-Earth model with just 15 Gha land surface, rather than the new estimate with terrain of at least 30 Gha. Thus, all past and current IPCC land estimates may be doubled, including their estimated “
combustion” burning of vegetation and detritus of 4 Gt C/yr, now presumed at around 8 Gt C/yr. Data values from IPCC (
2001: fig. 3.1 WG1-TAR1), as presented by Houghton (
2007: fig. 1), are updated in the following figures (
Figure 5 and
Figure 6).
SOC stocks may be broadly sub-divided between mineral soils, Permafrost, peats, plus biota. Compare data in Fig. 6 left-side to Canadell et al. (2021: fig. 5.12) having Soils 1,700 Gt (actually now >4,100 Gt) and Permafrost 1,200 Gt (actually now >2,000–5,000 Gt SOC plus extra in peats), to give their total of just 2,900 Gt SOC, plus Vegetation 450 Gt (now closer to 1,100 Gt) with an export of 2.5 Gt C/yr to rivers and 0.8 Gt C/yr from rivers to the sea. They also have gross photosynthesis on land of 142 Gt C/yr implying NPP = 71 Gt C/yr (while it is truly closer to 218 Gt C/yr from current data) with their total plant/soil respiration and fire emissions of just around +136.7 Gt C/yr (cf. new values in Figs. 5–6).
An earlier ESSD (2019: fig. 2) had mineral Soils ranging 1,500–2,400 and Permafrost as 1,700 Gt C to total up to 4,100 Gt SOC (about the same as combined total of all their Fossil Fuels + Atmospheric + Ocean organic + Vegetation carbon of ~4,150 Gt C). An estimated “Vegetation” range was 450–650 Gt C (median 550 Gt). Strangely, the latest ESSD (2022: fig. 2) now has Permafrost just 1,400 Gt C, Soils with 1,700 Gt C and Vegetation just 450 Gt C (total 3,550 Gt?). SOC in mineral soils, “officially” around 2,000 Gt C, when doubled for depth and/or terrain is ~4,000 Gt C plus neglected glomalin may be added. Glomalin is rarely considered, seemingly it alone increases mineral soil SOC values up to 20–30% (Comis 2002, Irving et al. 2021), further increasing with depth (Wang et al. 2017).
A small “inert” component, as in Fig. 5, is likely comprised in part of charcoal from Fire as in Fig. 6. In contrast to many artificial “char” projects, Deluca & Boisvenue (2012) discuss importance and age of naturally-occurring wildfire charcoal in C inventories.
Figure 5 and
Figure 6 may be further compared to an isotope analysis by Alves et al. (
2018: fig. 3 from Bolin & Fung 1990: fig. 1) that, while failing to mention “
soil” in the text, has land flux values with carbon inventories and rates considerably lower than current estimates (viz. Blakemore
2018b, 2020c and herein) yet presents similar soil gain/loss pathways at >3–7.0 Gt C/yr lost to ocean or air from soil/litter/peat degradation and net 0.4–2.6 Gt C/yr “
Anthropogenic flux” in CO
2 emissions from LUC. All values may be doubled for terrain.
Regarding Soil Inorganic Carbon (SIC) and Dissolved Organic Carbon (DIC) these add to soil carbon tally and often have biotic origin, especially carbonates (e.g., CaCO3 as noted in Fig. 5). To just 1 m soil depth, Monger et al. (2015) have “inorganic carbon as soil carbonate (~940 PgC) and as bicarbonate in groundwater (~1404 PgC)” and Lal (2019: tab. 1; 2020) has “global SIC (primary and secondary carbonates) stock is estimated at 1237 Pg to 2-m depth [plus an extra 321 Gt in humic soils] and an additional 1404 Pg as bicarbonates stored in ground water”. This value of ~2,962 Gt, if doubled for terrain, is >5,924 Gt SIC/DIC. Sizeable portions of CaCO3 on Land have biotic origins, some derived from earthworm calciferous glands is partly extracted from atmospheric CO2 (Blakemore 2019c - vermecology.wordpress.com/2019/11/11/earthworm-cast-carbon-storage-eccs/) as a valid “CCS” that alone is much greater than proposed BECCS/CCS sequestration schemes.
While Reiners (1973) had estimated SOC in “terrestrial detritus” to total 9,120 Gt C, most initial starting values quote just >1,500 Gt SOC, yet latest data from Wang et al. (2022: tabs. 1, S3) of flat ~1-km grid WISE vs. SoilGrids data in top 2 m are 2,814.8 vs. 5,796.1 Gt SOC. Terrain and/or depth may more than double these values to range >5,630–11,592 Gt SOC as reasonable new total global values. [Contrarily, FAO/GSP (2022) still publish just 1,500–3,000 Gt total SOC they claim includes all litter, roots, biota, etc.].
Global SIC/DIC values now up to 6,000 Gt C add to SOC/DOC (>10,000–15,000 Gt) and biota (2,000 Gt) to give total soil Carbon stocks of around 18,000–23,000 Gt C.
3.2. Biotic SOC Stocks Justified in Further Detail
Terrain consideration is not the only justification to revise SOC/SIC or DOC/DIC totals. Most current estimates are based on superficial soil layers, overlook litter/logs, roots, biota and/or peats and surprisingly undervalue or quite ignore Permafrost.
3.2.1. Biotic Boreal Permafrosts Reconsidered but Not Reconciled
Permafrost is a massive and ancient soil carbon store reaching depths of 1.5 km and extending under the Ocean (out of Land’s domain?). Permafrost soils “officially” contain 1,460–1,600 Gt C in surface 0–3 m including in Yedoma regions or river deltas, plus ~501 Gt C in other deep terrestrial sediments and the Qinghai-Tibet Plateau, along with about ~560 Gt C subsea from the Pleistocene (Schuur et al. 2015, 2022: 348). Excluding ~560 Gt in subsea, a total is thus 2,101 Gt that terrain may double to >4,200 Gt SOC. Significantly, these latter authors compare their new totals to “the 2,050 Pg C of organic soil C (from 0- to 3-m depth) contained in all other biomes” that, similarly doubled, is ~4,100 Gt SOC, or about equal to the total terrain Permafrost SOC value, as indeed is presented in the Abstract.
Moreover, due to melting, these authors estimate 67–237 Gt C emissions by 2100, or ∼0.5–2 Gt C/yr loss, stating: “carbon release from [permafrost] soils to the atmosphere could outweigh the potential for carbon gain by plants”. If also doubled, this loss is ~1–4 Gt C/yr.
Raupach & Canadell (2010) earlier estimated Permafrost with 1,700 Gt C of which around 100 Gt C (= ~50 ppm CO2) was vulnerable to release by thawing over the next century. Predicted warming trends within the circumpolar region could result in release of 30–60 Gt C by the year 2040 according to Deluca & Boisvenue (2012). Trubl et al. (2018) have Permafrost thawing at a rate of ≥1 cm of depth per year also releasing many microbes. Permafrost soils and peats are many millennia old; their carbon may date to >2.6 million years according to a recent Nature paper on 1.6 million year old preserved mammoth DNA (Callaway 2021). With 14C half-life of 5,568 years (Alves et al. 2018: fig. 2), such aged organic soil has its 14C decayed by 280–450 half-lives thus its loss to the atmosphere would have similar isotope profile as do fossil fuels. Shi et al. (2020) summarized: “Integrated to a depth of 1 m, global soil carbon has a mean age of 4,830 ± 1,730 yr, with older carbon in deeper layers and permafrost regions. In contrast, vertically resolved land models simulate ∆14C values that imply younger carbon ages and a more rapid carbon turnover”.
Notwithstanding general terrain considerations, Permafrost SOC tally yet has large uncertainties and is widely underestimated in its neglected hill-slope bases (hill toes) by >200% having new mean values ∼550 and ∼720 Gt SOC “for the linear and sigmoidal profile geometries, respectively, with a maximal uncertainty of >2000 Pg C” (Shelef et al. 2017). Adding to prior boreal Permafrost totals would then possibly represent 1,700 + >2,000 = >3,700 Gt C (to 3 m depth?) or may extrapolate to as much as 1,700 x >200% = >5,100 Gt SOC.
Deluca & Boisvenue (2012) reported: “The expansion of temperate and boreal forest ecosystems back into glaciated landscapes resulted in the net accumulation of 500–1350 Pg of C on the Earth’s surface”; presumably a large part of this added to Permafrost and peat carbon? These large carbon stores are likely both expanding and simultaneously melting as temperatures rise disproportionately rapidly in the far North. It is important to note that global average temperature rises are about twice as high on land as in the oceans and about doubled again in the boreal North with the tangible risk of self-fueling Permafrost melting and/or peat fires causing cascading (snowballing?) climate warming effects.
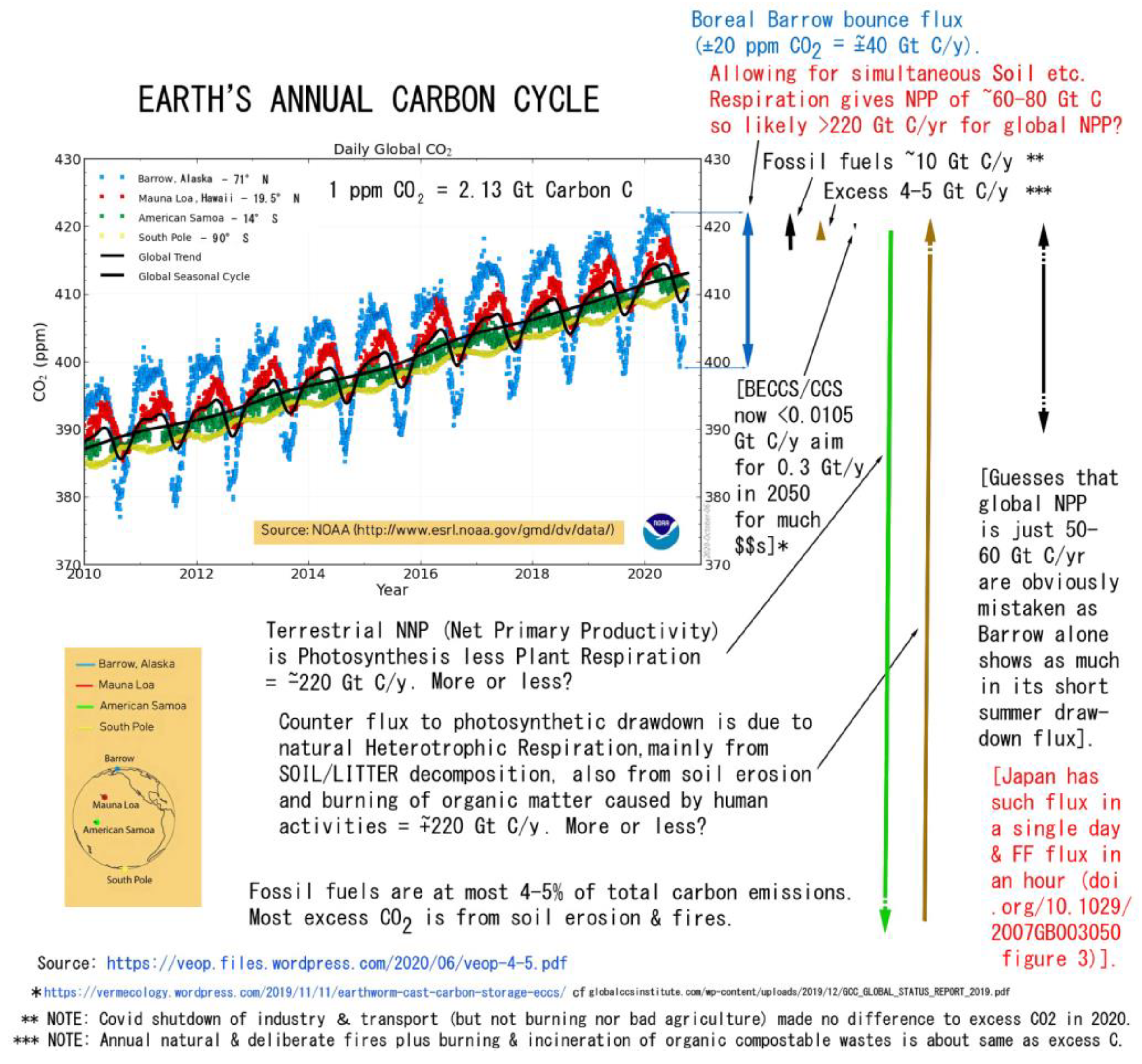
Recently, in June 2020 in the midst of uncontrollable peat fires, the boreal North experienced record air temperatures of 38°C that were +18°C above their average, and the Arctic has already warmed to more than 2°C above the Preindustrial level with this rapid warming expected to double by midcentury and potentially emit up to about 150 Gt C by 2100 (Natali et al. 2021). Thus, a 1.5°C global rise may be more than twice this on land and higher yet for soil, more so further North. Globally: “mean annual soil temperature differs markedly from the corresponding gridded air temperature, by up to 10°C (mean = 3.0 ± 2.1°C), with substantial variation across biomes and seasons” (Lembrechts et al. 2021). Thus, if air temperature is +2°C, soil may go up +5°C. (Summaries are to be found here: https://earthobservatory.nasa.gov/images/146879/heat-and-fire-scorches-siberia; https://vermecology.wordpress.com/2020/08/31/barrow/; https://vermecology.wordpress.com/2022/05/25/2022-year-of-the-taiga-barrow-2/).
3.2.2. Missed Peats
Peats contain more carbon than above-ground vegetation, including the World’s forests. In spite of this, peatland ecosystems are still omitted from the main Earth system models that are used for future climate change projections or mitigation schemes according to Loisel et al. (2021). These authors (tabs. S4–5) show peat stocks of 808 + 315 = 1,123 Gt C and predict 100 Gt C could be lost by 2100, although large uncertainties remain (www.exeter.ac.uk/news/research/title_829842_en.html). Sometimes included in carbon inventories, sometimes not, carbon-rich peatlands cover ~2–3% of land area yet contain about 10% of global SOC with degradation from drainage, fires or exploitation of at least 3 Gt CO2 per year (equivalent to ~0.81 Gt C/yr) or ~10% of the global fossil fuel emissions according to Parish et al. (2008: tab. 9.1). Peat total was recently doubled from 550 Gt to 955–1,060 Gt by an extra ~510 Gt overlooked in northern peatlands (Nichols & Peteet 2019, 2021). So peat loss may now be doubled too to ~1.62 Gt C/yr? Waterlogged peat does not gain as much from terrain, however peat values are seemingly only for a depth of 1 m and Parish et al. say: “The deepest peat/lignite layer in the world is probably the Phillipi peatland in Greece, reputed to be 190 m deep... and dating largely from the Pleistocene”. Thus, values may yet be increased two or more times for depth. Moreover, peats can also be ancient (“peatlands have certainly existed for hundreds of millions of years”), again diminishing isotopic C dilution argument solely from the burning of fossil fuels (e.g., coal, oil, gas).
3.2.3. Essentials of SOC Stocks from Above- and Below-ground Biota
Above-ground vegetation and below-ground biota tallies were stated by Crowther et al. (2019;
https://www.osti.gov/servlets/purl/1559650): “
Soil is the largest repository of organic matter on land, storing ~1500 Gt carbon, which is at least as much as the vegetation (~560 Gt) and atmosphere (~750 Gt) combined.” Unfortunately all three values were mistaken since soil and vegetation totals were already >10,000 Gt C and >1,000 Gt C, respectively (Blakemore 2018b), and, according to IPCC and ESSD (2019: fig. 2), atmosphere had 860 Gt C then (now 875 Gt C from ESSD 2022: fig. 2). Moreover, Blakemore (2020c: fig. 1
https://orgprints.org/id/eprint/38139/1/Veop-4.pdf) summed Crowther et al. (2019: fig. 2C) soil grid values to 4,595 Gt C, not 1,500 Gt as they stated. (Their SoilGrids data source now totals 5,796.1 Gt SOC, according to Wang et al. 2022). Doubled for terrain, land easily supports ~10,000 Gt SOC. Crowther et al.’s plant and microbial biomass actually added up to ~595 and 23 Gt C, respectively (not ~560 and 7.5 Gt C as claimed); both presumably also doubled for terrain to ~1,191 and 46 Gt C. Although the corresponding author declined to reply nor self-rectify these errors (pers. comms.), corrected information was detailed already (R. Blakemore 2019d
Science eLetters commentary DEC. 2, 2019- science.org/doi/pdf/10.1126/science.aav0550). Appropriately revised and corrected, values showing interesting geographic distributions are provided below (
Figure 7).
Compared to
Figure 7, Rodin et al. (1975: tab. 1) had ~1,200 Gt C “
phytomass cover” although these authors assumed a pre-cultivated or natural state and possibly included roots. Bar-On et al. (2018: tab. S1) for “
Trees” had 450 Gt C, ESSD (2019, 2022) Vegetation values ranged 450–650 Gt C (mean 550), while Wuepper et al. (with T. Crowther) (2021) “
above-ground” plant biomass was 601 Gt C, itself above Crowther et al. (2019) value of ~560 Gt C. Such large inconsistencies require convergence. Nevertheless, realistic final above-ground vegetation estimates, doubled for terrain, are likely around ~1,100 Gt C.
3.2.3.1. Roots
Roots spread deep and wide for many plants, for example, prairie grasses or trees with deepest known at 68 m for Boscia albitrunca (Burch.) in Botswana’s central Kalahari desert (Canadell et al. 1996). A summary for roots biomass by Blakemore (2018b) had reported an initial 146 Gt C from a dry biomass of 292 Gt from Jackson et al. (1997: tab. 2) that was allocated about 175 Gt (80%) for forests and about 42 Gt (20%) in other biomes. Jackson et al. found fine roots alone (also ~20% of their total) representing 33% of total annual net primary productivity (NPP). Roots total, as updated by Mokany et al. (2005: 95) to 241 Gt C, is less than an earlier figure of 267 Gt C by Robinson (2004: fig. 2) he said comprised about half of 492 Gt C estimated for the planet’s above-ground vegetation. Robinson’s initial value seems mainly for tree roots rather than for grasses, scrub, tundra, desert, etc. that Mokany et al. and Jackson et al. (1997: tab. 2) show as substantial (~20%).
Robinson (2004) found that true below-ground biomass of tree roots in general are not only underestimated by about 60%, but also that losses as large as 20–40% of root samples can occur after recovery from soil due to subsequent handling, washing and storage; i.e., errors may amount to 100%. Therefore, instead of an initial 160 Gt C as then estimated in (tree?) root systems globally, he said a true amount could be about 267 Gt C. If 20% of this value is added for other than forest biomes (from Jackson et al. 1997: tab. 2), a new total is about (267 + 53 =) 320 Gt C. Alternatively, a new total from Mokany et al.’s (2005) 241 Gt C with 60% added for missed tree roots, plus mean 30% added for Robinson’s sampling errors, is (241 + 217 =) 458 Gt C. Doubled for terrain this then is 916 Gt C, or roughly the same as above-ground plant biomass previously estimated (~1,100 Gt C).
Some support for this is Qi et al. (2019) global synthesis of a root:shoot ratio of 0.90. Often commensurate, ratios for trees are lowest with grasses highest and Reiners (1974: tab. 4) footnoted roots as high as 74% of total phytomass for Calluna heathland in UK.
Dead or decaying roots form an integral part of a litter pool but are rarely included.
3.2.3.2. Litter, Logs and Roots in Above- and Below-ground “Detritus”
Theoretically, all ~220 Gt C/yr shoot and root NPP is eventually converted to litter detritus that is recycled by earthworms/microbes (e.g. Guo et al. 2021). Brovkin et al. (2011) proposed a (flat) global litter amount of 184 Gt C noting it was towards upper ranges of “litter stocks based on observations (68–97 Gt C) or models (47–196 Gt C)”. IPCC (2001: fig. 3.1d) and Houghton (2007: fig. 1) had about 550 Gt C in “Vegetation” and 300 Gt C of “DETRITUS” plus 1,500 Gt C total soil organic matter (cf. just 3 Gt C in their Ocean “Surface biota”) (see vermecology.wordpress.com/2022/07/04/ip-bees/). Their 300 Gt C in soil litter (and logs?) was possibly derived from Matthews (1997) litter report of (160 fine + 150 coarse =) 310 Gt “dm = dry matter” with “rarely included” belowground litter. This value should properly be halved for carbon to 155 but doubled again for terrain to 310 Gt C. Matthews also noted “including standing and fallen dead wood may increase estimates of the fine litter pool by ~40%”. Compare this to Reiners (1973 from Bolin 1970) that has humus, mulch, etc. “detritus” at ~700 Gt C. Yet, if reasonably assuming Houghton’s 300 Gt C value was correct, as also adopted herein, the total may be updated and doubled for terrain to about 600 Gt C as per Blakemore (2020a, 2020c: fig. 3) and as shown in Figs. 5–6.
3.2.3.3. Contributions of Soil Biota (Biodiversity, Biomass & Necromass) to Total SOC
Global biomass data from Bar-On et al. (2018: tab. S1 - rpdata.caltech.edu/publications/Bar-On_2018_SI.pdf) were updated in Blakemore (2018b: fig. 12, tab. 10) and Blakemore (2022). Note that Bar-On et al. (2018: tab. S1) “Trees” at just 450 Gt C, plus soil Bacteria, Fungi, and Protists at 24 Gt C, totaled >474 Gt C on Land (>99%) while Ocean had ~3–6 Gt C ~(1%). Terrain and other correcting adjustments, as detailed herein, up soil biota values to >230 Gt C plus Phytomass (shoots, roots, litter/logs) now total 2,880 Gt C or >99.9%, thereby reducing Ocean share to <0.1%.
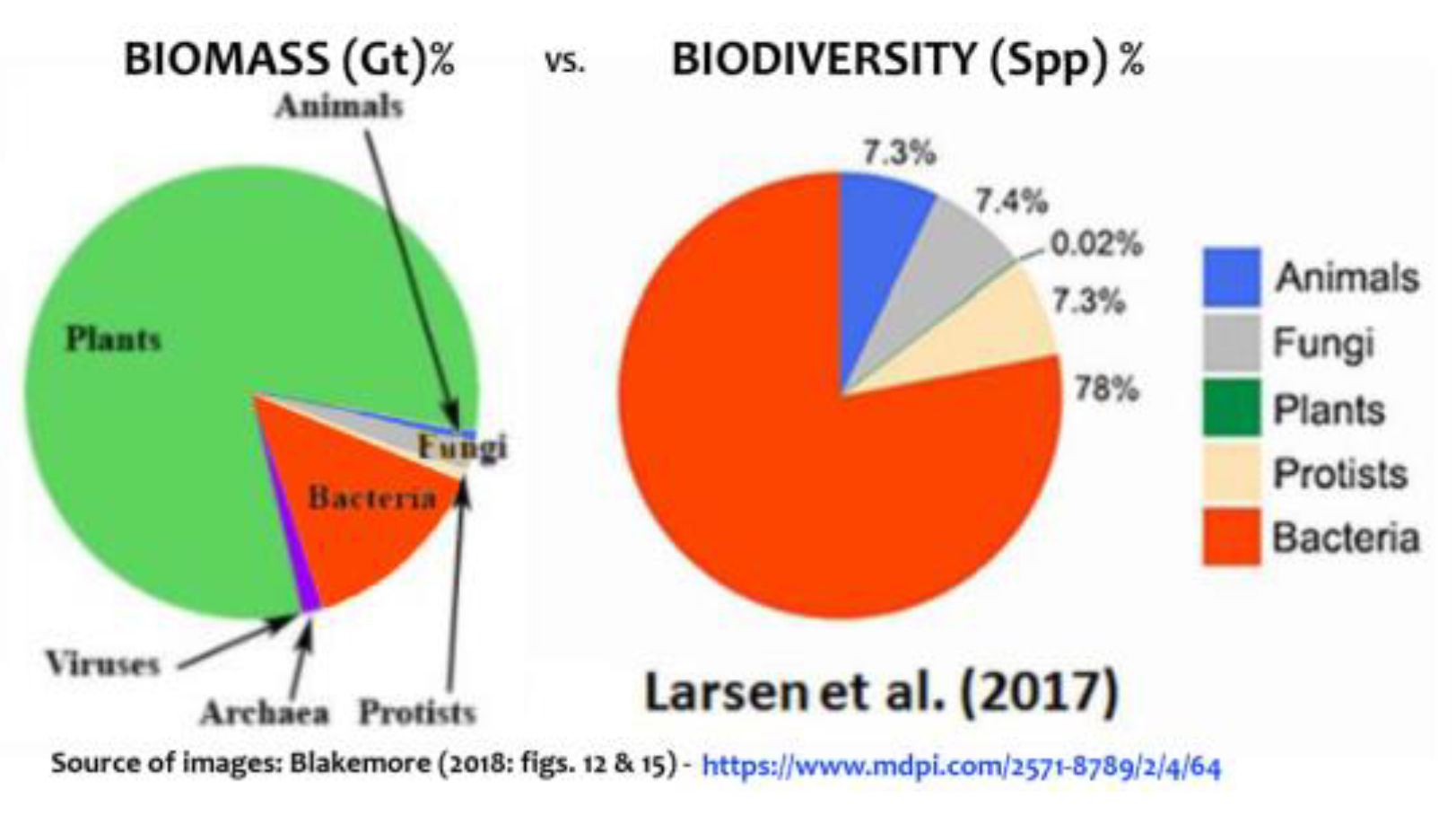
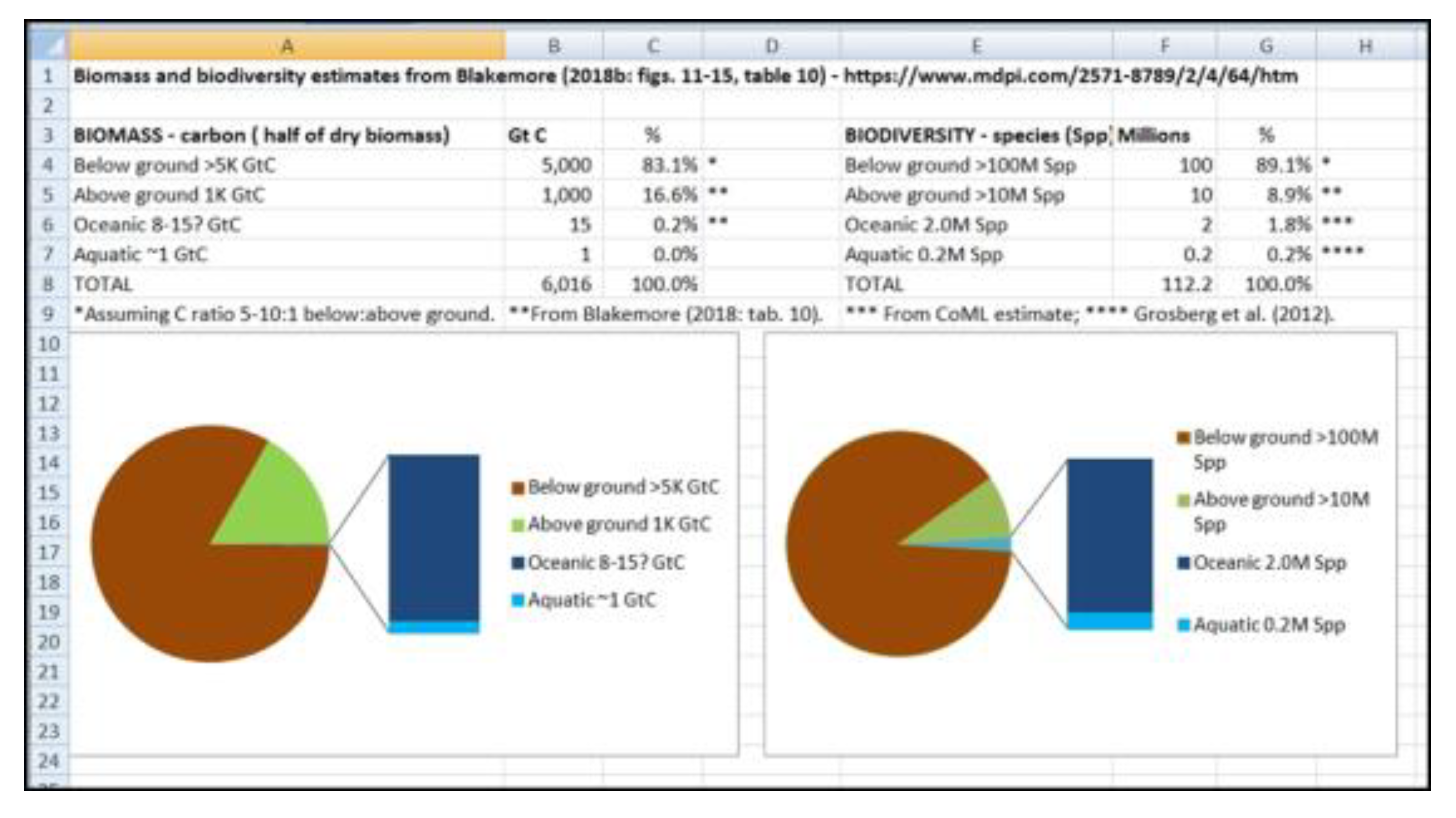
Vermeij & Grosberg (2010) estimated between 85% and 95% of all living macroscopic species are found “on land”, while Locey & Lennon (2016: fig. 3) showed Earth with ~1012 microbial original taxonomic units (OTUs) with just 1010 or ~1% in global Oceans. When microbes are fully accounted for, soil alone supports >99.9% biodiversity with 2.1 x 1024 species (Blakemore 2022). This is consistent with UNEP (2002: 10) finding that probably over 80% of total plant production soon enters the soil system either through plant roots or as leaf-litter and perhaps 50% of below-ground allocation is released as extra-root carbon exudates, some being ‘traded’ with microbes for Nitrogen fixation or other growth factors. Intricate soil biotic pathways include microbial necromass and products that make up as much as 80% of global SOC according to Gross & Harrison (2019: fig. 1). Bar-On et al. (2018: tab. 1) have 70 Gt C Bacteria, mainly terrestrial, with a 10-fold uncertainty, presumably giving a biomass range of 7–700 Gt C. They claim an above-ground plant biomass (≈320 Gt C) represents ≈60% of global total, with below-ground biomass composed mainly of plant roots (≈130 Gt C) to total 450 Gt C in “Plants”. Total microbes residing in soil or deep subsurface they give as ≈100 Gt C. Terrestrial Arthropods (mainly insects or similar), Annelids and Molluscs they have at just 0.2 Gt C for each while total soil Protists are 4 Gt C (to give total soil organisms just 4.6 Gt C?). (For marine biota they have a generous ≈6 Gt C). All these values (except for those in water) are revised and at least doubled for neglected terrain considerations herein. Figures below summarize progressive biomass and biodiversity reviews (Figs. 9–11).
Figure 10.
Biomass and Biodiversity reallocations sourced from Blakemore (2018b: figs. 12 & 15).
Figure 10.
Biomass and Biodiversity reallocations sourced from Blakemore (2018b: figs. 12 & 15).
Figure 11.
Early estimates with large disparity in global Biomass and Biodiversity allocations for main Realms-of-Life (excluding the atmospheric “Aeolian biota” or Aeliota that likely supports as much life at any time as the Oceans & Aquatics combined, as in Tabs. 1–2). Source: https://vermecology.wordpress.com/2022/03/29/eco-taxo-bio/; https://vermecology.wordpress.com/2022/07/04/ip-bees/. Soils support an estimated >83% of biomass (arguably 99.7% if the above-ground biota are included) and >99.9% biodiversity with latest microbial data (Blakemore 2022; https://vermecology.wordpress.com/2022/08/04/different-f3/).
Figure 11.
Early estimates with large disparity in global Biomass and Biodiversity allocations for main Realms-of-Life (excluding the atmospheric “Aeolian biota” or Aeliota that likely supports as much life at any time as the Oceans & Aquatics combined, as in Tabs. 1–2). Source: https://vermecology.wordpress.com/2022/03/29/eco-taxo-bio/; https://vermecology.wordpress.com/2022/07/04/ip-bees/. Soils support an estimated >83% of biomass (arguably 99.7% if the above-ground biota are included) and >99.9% biodiversity with latest microbial data (Blakemore 2022; https://vermecology.wordpress.com/2022/08/04/different-f3/).
3.2.3.4. Microbial Biomass
Zhao et al. (2022) rightly concluded: “soil is the most microbiologically abundant (∼1029) and diverse (∼1011) environment on the Earth” which translates into substantial biomass. To a depth of 1 m total soil microbe biomass in Bar-On et al. (2018 : tab. S1 pnas.org/doi/suppl/10.1073/pnas.1711842115/suppl_file/1711842115.sapp.pdf) was ≈20 Gt C comprised of Bacteria and Achaea of ≈8 Gt C and a total soil fungi value of ≈12 Gt C. Their soil microbial error margin was 10-fold (i.e., possible biomass range 2–200 Gt C?).
For soil Protists globally, Bar-On et al. estimated ≈1.5×1022 ciliates, ≈4×1023 testates, ≈1.5×1024 naked amoebae, ≈3×1025 flagellates; these numbers may be doubled for terrain. Their biomass amounted to ≈1.5 Gt C that, if also doubled for terrain, is about 3 Gt C.
Mainly for bacteria, Blakemore (2022) summarized 108–1012 microbial cells/g or 1014–1018 cells/t (since 106 grams/tonne). While Whitman et al. (1998: tab. 2) had 123 x 106 km2 soil with 1.3 t per cubic meter bulk density, perhaps a more modest flat "habitable land" is ~104 × 106 km2 (Blakemore 2018b: fig. 4, tab. 5). Doubled for terrain, to 1 m depth is ~208,000 Gt or ~2.1 x 1014 t global topsoil, leading to a new soil microbe total of 2.1 x 1028–1032 with a median value ~2.1 x 1030 cells (with 2.1 x 1024 spp), as is reported herein.
Soil microbe cell dry biomass Whitman et al. (1998) took as 2 x 10-13 g/cell with half carbon (and C:N = 1:0.24), thus, 2 x 10-13 g/cell x 2.1 x 1030 cells is 4 x 1017 g or 400 Gt (= 200 Gt C : 48 Gt N). (This dry biomass g/cell seems acceptable e.g., Sanz-Jimenez et al. 2022). Bar-On et al. (2018) said: “≈98% of the total microbial biomass is found in the top 1 meter of soil” further validating new microbial estimates to 1 m depth of global soils. Biota is dominated by Bacteria then Archaea with minor contributions (<1–2 %?) from other microbes with a biomass estimate herein coinciding with Bar-On et al’s. upper range of 200 Gt C.
3.2.3.5. Fungi
Earthworms aside, Fungi are probably the next most important soil organisms. From Bar-On et al. (2018: fig. 5) Fungi were 12 Gt C and 1027 cells, mainly in soils and herein at least doubled for terrain to 24 Gt C and 2 x 1027 cells. Contributions of Ectomycorrhizal fungi biomass they estimated as “roughly ≈0.2 Gt C” and for Arbuscular mycorrhiza (AM) “≈2 Gt C”. Rather than 12 Gt C as Bar-On et al. claim, Robinson (2004) found 15 Gt C in mycorrhizal hyphae alone, doubled for terrain gives soil fungi >30 Gt C.
3.2.3.6. Phytomenon and Biocrust
Soil phytomenon, surface biocrust, or autotrophic biofilm and some epiphytes (e.g., bryophytic liverworts, hornworts, epiphytic mosses plus microfungi/yeasts, photosynthetic green algae, lichens, and Cyanobacteria or Cyanophyta) coat the convoluted superficial and interstitial surface rocks, topsoil, or sands, and snow or ice. According to Elbert et al. (2012), these ‘cryptogamic covers’ or biocrust total about 5 Gt C taking up about 4 Gt C per year in NPP, but terrain doubles these values at finer scale to 10–20 Gt C and ~8–16 Gt C/yr (Blakemore 2018b; also - https://vermecology.wordpress.com/2022/08/04/different-f3/). Elbert et al. (2012) further derived a nitrogen uptake by cryptogamic covers of around 49 Tg per year (now >100–200 Tg N/yr), they say suggests that cryptogamic covers account for nearly half of biological nitrogen fixation. At >100 Tg N this is approximately the same as an annual oversupply of synthetic N fertilizer, as is noted below in an agronomic section.
Aside from soil cryptogams, Jassey et al. (2022) report an average 5.5 x 106 photoautotrophic algae per gram of surface soil (cf. autotrophic marine cyanobacteria and Prochlorococcus spp. density of 4 x 104 cells per ml seawater from Whitman et al. 1998), and soil algae alone having NPP of ~3.5 Gt C/yr that, when increased for micro-terrain, is likely also doubled or quadrupled in value. Soil’s phytomenon NPP is thus about the same – or possibly more – than all the Oceans’ phytoplankton NPP (of just ~20 Gt C/yr).
3.2.3.7. Earthworm Abundance, Biodiversity, Biomass & Ecological Activities
Earthworms (Annelida: Oligochaeta: Megadrilacea) are a major component of healthy soils (along with Fungi) due to intimate associations. Earthworms, plants, and soil microbes co-evolved interdependence, their interactions regulate Soil Ecology. Megadrile earthworms comprise 20 families, approximately 600 genera (several unnecessary sub-genera) and ~7,000 described species or sub-species with an expected total of over 35,000 species (Blakemore 2012, 2016a). They represent up to 90% of invertebrate biomass present in soil. Moreover, microbes increase during digestion and after gut passage in their fresh castings by up to x 1,000 (Lee 1985: 27, 206) further enriching soils.
Beneficial earthworm activities of micro-mixing, aeration and drainage stimulate microbial and other soil-faunal activities thereby enhancing plant growth (
Figure 12).
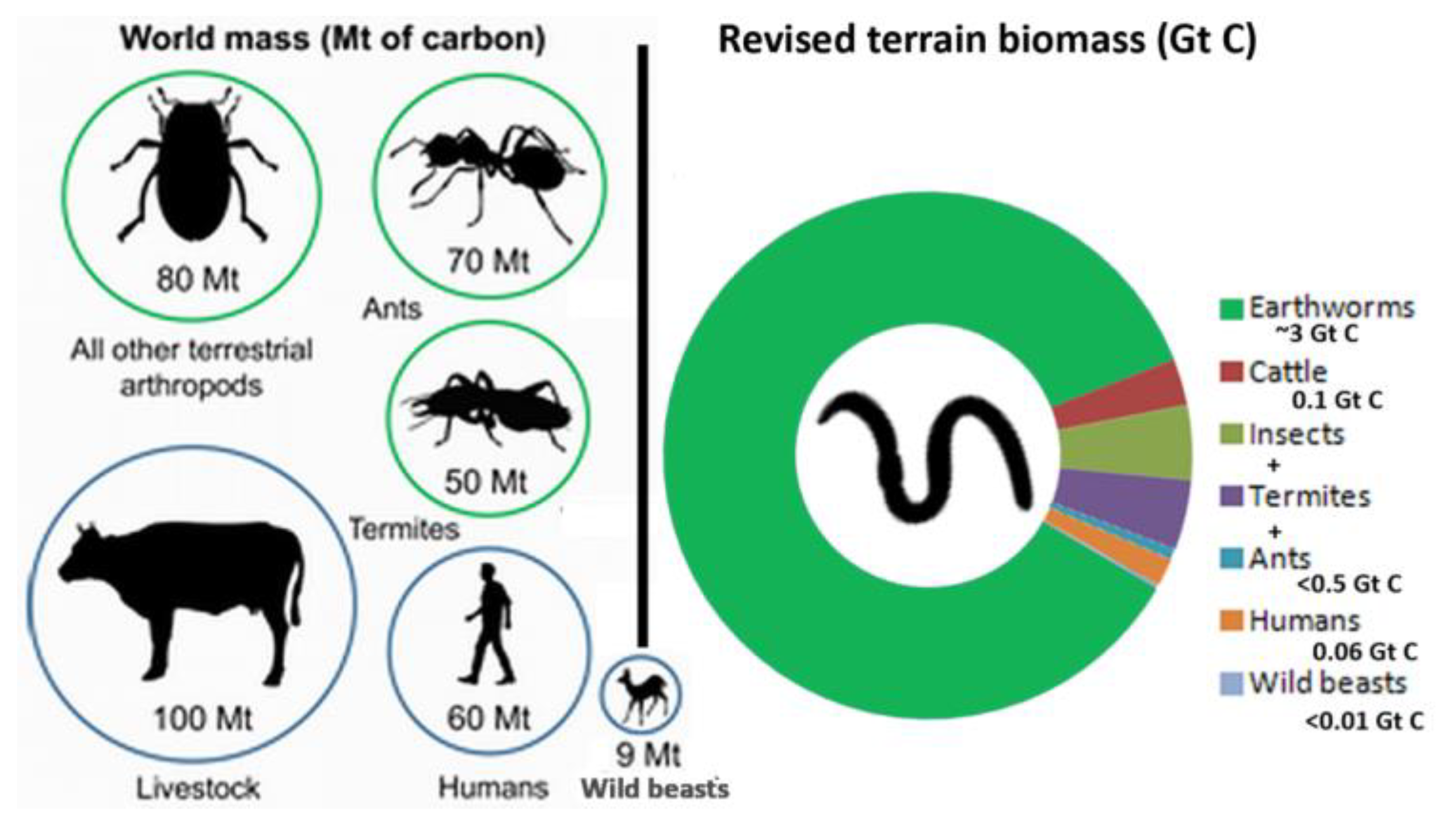
His professional life mostly spent studying Earthworm Ecology, Charles Darwin (1881: 158) calculated from Hensen (1877: 360) that there must exist 133,000 living worms in a hectare of productive land (ca. 13.3 /m2) with 3 g per worm (Darwin mistakes this for 1 g) (= 40 g/m2). In previous reports these were multiplied by “flat” habitable land areas of ~10 Gha to give totals of about 1.3 x 1015 or 1.3 quadrillion, with 4.0 Gt live biomass.
From a wider range of habitats, including alpine, taiga, and dry sclerophyll scrub, an average 273 worms/m2 (= 2.73 x 106 ha-1) and a fresh wt biomass of 63 g/m2 (= 0.63 t ha-1) were derived from Lee (1985: tab. 7) and multiplied by realistic land area (Whitman et al. 1998: tab. 2) including scrub, tundra, alpine, etc., of 12.1 Gha by Blakemore (2000, 2016a, 2018b) to give 32.8 x 1015 worms and 7.6 Gt live weight – almost double Darwin’s figure (vermecology.wordpress.com/2017/02/12/nature-article-to-commemorate-charles-darwins-birthday-on-12th-feb/). Doubled for terrain these now are 65.6 x 1015 worms and 15.2 Gt wet mass. At 30% moisture (Lee 1985: 33), dry mass is 4.5 Gt with half carbon at ~2.3 Gt C.
For “Annelida” (Oligochaeta comprising Megadriles + Microdriles with marine Polychaeta explicitly excluded) Bar-On et al. (2018: 31–32) had “300 individual earthworms per m2 ”, 5 mg C per worm and 1.5 g C/m2 (i.e., 3 g dry, 10 g wet/m2), supporting my abundance numbers of 273 /m2 and fresh ~63 g/m2. However, their 0.2 Gt C total earthworm biomass is now raised to ~2.3 Gt C (Blakemore 2022). Ratio C:N 1:0.24 gives 0.54 Gt N or 540 Tg N, five times more than synthetic Nitrogen added to farmlands per year(!).
In wet organic soils, microdrile Enchytraeidae may range 103–105 per m2 (Adl 2006) two to three times megadrile 1015–1016 totals, so total Oligochaeta range 1017–1019 or median 1018. Bar-On et al. (2018: tab. S1) megadriles + microdriles total of 1018 is thus feasible.
Enchytraeidae population maxima of 290,000 /m2 (~3 x 105 /m2 and 53 g/m2 live) were in peat at Moor House Nature Reserve in England (Gragg 1963: tab. 2, Springett 1967: fig. 24) while limestone grassland plots had 389 earthworms /m2 (110 g/m2 live weight).
One of the highest megadrile records is of Pontodrilus litoralis (Grube) that Coupland & McDonald (2008) reported with populations of 750–4,875 /m2 under wrack seaweed on arid beaches in WA they calculated consumed 19–31 kg/m2/yr organic material. Highest earthworms in Lee (1985: tab. 7) were in NZ pastures (2,020 /m2 with 305 fresh g/m2 from McColl & Lautour 1978). Another example, in a 1,000 year-old permanent pasture in the UK where six lumbricid species attained 456 /m2 and 153 g/m2 (Blakemore 1981, 1994, 2000, 2016a, 2018a). A pasture population in NZ of 716 worms/m2 (160 g/m2) extrapolated to a field population of 7.16 million worms ha-1 and 1.6 t/ha (Blakemore 2011). Such biomass exceeds all other soil fauna, matching above-ground cattle or sheep stocking rates.
Eve Balfour (1943) gave Rothamsted earthworm counts of 123 /m2 in unmanured plots (as for N-P-K plots); 680 /m2 in FYM plots; 2,125 /m2 on grassland (cf. Blakemore 2018a: tabs. 1–7). She also pointed out that “Earthworms render soil permeable to rain thus checking the tendency to erosion by rain and wind. Aeration and nitrification are also stimulated”.
Overall megadrile means in Lee (1985: tab. 7) of 273 /m2 and 63 fresh g/m2 give an average mass per worm of 63/273 or about 0.23 g/worm. However these figures are low estimates when earthworms in fertile soils, forests, or orchards are more likely in the order of 50–500 /m2 and 20–400 g/m2 (Blakemore 2016a: tab. 2). Thus, potential ideal maxima are of 500 worms/m2 and 400 g/m2 that, if in ~10 Gha fertile soils are 50 x 1015 and 40 Gt (12 Gt dry, 6 Gt C). Doubled for terrain to 24 Gt dry and 12 Gt C is tempered with ~69% earthworm biomass decline under intensive agrichemical regimes (Blakemore 2018a: tab. 11) thereby likely reducing this to just ~3.6 Gt C, as presented in the Abstract.
Conversely, simply conserving earthworms may reduce soil erosion and farm fertility, not least their burrows allowing better drainage and moisture holding capacity; they optimally construct ~9,000 km/ha increasing soil porosity, infiltration, and stimulating plant growth (Gaupp et al. 2015). Pimental & Kounang (1998) quote 220 worm burrows per m2 (3–5 mm diameter) which is 2.2 million drainage points per hectare, some that may extend for several metres depth. Worms may process their body weight of soil each day, or possibly ~1,314 Gt dry soil per year. Lee’s (1985) figures show they recycle all organic Ah soil horizon in 4 years (cf. Darwin 1881).
Agrichemical overuse has effectively eliminated earthworms from fields, pastures, or forests; at least three species are extinct many more likely also gone (Blakemore 2018a).
3.2.3.9. Soil Nematodes
Soil nematodes of 4.4 × 1020 expressed either as density per gram of soil or per unit area on a planimetically flat basis (van den Hoogen et al. 2019) with a total biomass of ~0.3 Gt translates to ~0.03 Gt C (which the authors claim is three times greater than a previous estimate of soil nematode biomass and represents 82% of total human biomass). Herein raised for neglected terrain, now to 8.8 x 1020 nematodes with ~0.06 Gt C biomass.
3.2.3.10. Soil Viruses
Regarding soil viruses especially bacteriophages – aside from questions (as for mitochondria) on whether they are living entities or not – their abundance in soils may be miscalculated by orders of magnitude. In Bar-On et al. (2018: tab. S1), global viruses are 1031 with biomass of 0.2 Gt C and 108–109 phages per gram of soil. Yet a summary paper (Trubl et al. 2018) found: “While many soils contain large numbers of viral particles (107 to 109 virus particles per gram of soil.. knowledge of soil viral ecology has come mainly from the fraction that desorbs easily from soils (<10% ..) and the much smaller subset that has been isolated.” If <10% are detectable, a likely range is then >108–1010/g and, as with soil bacteria, their numbers are massive and a reasonable mean estimate is of 109 viruses/g that, if a similar proportion is isolatable, may be closer to 1010 (coincidentally this is the same value provided by Kuzyakov & Mason-Jones 2018). Thus, Earth’s ~2.1 x 1020 g soil to 1 m depth may have ~2.1 x 1030 virions and, if to 10 m soil depth, = ~2.1 x 1031. So Bar-On et al.’s total 1031 at 0.2 Gt C, if doubled in soil alone equates to ~0.4 Gt C. Compare to Bar-On et al.’s (2018: 55): “assuming a mean soil depth of 10 meters (276), we get to an estimate of ~1029-1030 virions in soil” with mean ≈6.2 × 1029 soil viruses (mainly phages) having a 32-fold uncertainty(!). Terrain allowance may double their total to ≈1.24 × 1030, presumably then with one-tenth or ~0.02 Gt C. Mushegian (2020) confirms virome quadrillion-quadrillion (>1030) count, yet, as mentioned, may be out by orders of magnitude and whether this truly adds living organisms to an ever-evolving soil biota tally is up for debate.
Since compilation, new soil virus data is provided (www.soilviral.com/) having: “1 billion viruses g-1, that if calculated over the whole globe amounts to about 4.9 x 1031 soil viruses globally”. Doubled for terrain, this becomes 10 x 1031 and 4 Gt C as possible new values.
Of note is whether soil viruses impact SOC, nutrient cycling, emissions or NPP productivity as they do in marine settings where 20–40% of microbial standing stock lyse daily to a dissolved nutrient pool (Kuzyakov & Mason-Jones 2018). Williamson et al. (2017) discuss such issues and, in an cogent Conclusion, remark: “Soils represent the greatest reservoir of biodiversity on the planet; prokaryotic diversity in soils is estimated to be three orders of magnitude greater than in all other ecosystems combined… Soils remain the most poorly understood ecosystems on Earth. At the same time, viruses represent the largest pool of untapped genetic diversity and unexplored sequence space on the planet. In this regard, the soil virome comprises an unknown quantity within an unexplored territory: a vast new frontier, ripe with opportunities for discovery.” Thus the current report is not alone to realize such facts.
3.3. As Soil Biodiversity Soars, Extinction Rates Also Rise
Soil biodiversity from divers sources (as in Blakemore 2012: tab. 1, 2016a: tab. 3, 2022: tab. 5) had about 315,500 prospective soil species listed with biomass of ~1,500 Gt C (part in SOC). Arguably, this should include any plants that root or seed in soils as a factual component, thereby adding an extra ~500,000 taxa (Corlett 2016). This 815,500 species total was revised (Blakemore 2022) to update microbial biodiversity with latest genomic or other “Omics” estimations of taxa showing soil houses ~2.1 x 1024 taxa representing >99.99% of global biodiversity, mostly Bacteria or other soil microbes.
Justification was based upon 102 –106 unique species (spp) per gram of dry soil (or 108 –1012 spp/t), and Earth supporting ~2.1 x 1014 t of habitable soil, to give a reasonable range of 2.1 x 1022 –1026 spp with median value ~2.1 x 1024 soil spp. Fishman & Lennon (2022) had: “bacterial and archaeal taxa Spresent is between 106 and 1023.” The present ~2.1 x 1024 soil microbial species increased their upper value by a factor of about twenty-times.
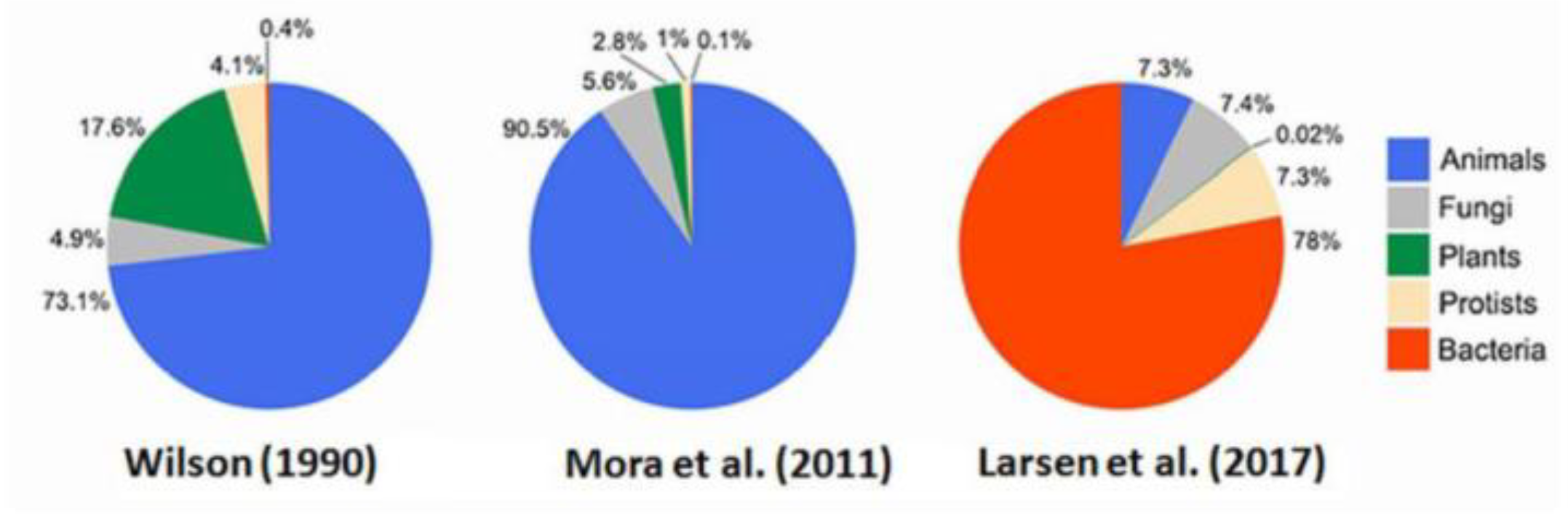
Such biodiversity far outnumbers the mere 2 million (10
6) currently described species, yet Larsen et al. (2017) speculated: “
Our estimates suggest that there are likely to be at least 1 to 6 billion species on Earth [
109]
. Furthermore, in contrast to previous estimates, the new Pie of Life is dominated by bacteria” while Locey & Lennon (2016: fig. 3) show a trillion (10
12) microbial taxa, just 10
10 (<1%) in the Ocean.
As biodiversity estimates climb, actual on-the-ground species decline due to rapidly increasing extinctions, up to 100–1,000 x above expected rates (IPBES 2019: fig. SMP3: “background rate of 0.1-2 extinctions per million species per year”). IPBES lacks context or triage, thereby losing some credibility, appearing to give equal weight or status to land:sea:freshwater when in factual reality these respectively provide 99.9:0.1:0.0% to biodiversity or human survival, as is shown in the Introduction, in Tabs. 1–2, and Fig. 10.
This is a large and complex topic but some key references are E.O. Wilson’s (1992) prediction from rain forests of 27,000 extinctions per year (74 per day), and IPBES (2019) reportedly having a rate up to 200 species lost per day, mainly on land and mainly due to LUC. If massive new biodiversity estimates have similar proportional rates (currently up to 200 lost per day, but just large charismatic taxa not the 99% of lesser invertebrates so a true base rate may be 100 x higher at 20,000 per day?), this may increase 100s x again for >90% microbes in >1 billion taxa. Conservatively, this is 2 million species per day or 23 taxa per second (vermecology.wordpress.com/2021/06/20/tol/) as in the Abstract.
Just one example for microbes is Streptomyces avermitilis (ex Burg et al.) that was found only once in a soil sample collected in 1977 near a golf course at Ito, Shizuoka-ken. For terrestrial invertebrates, Regnier et al. (2015) estimated critical 7% species loss while Cowie et al. (2022) had 7.5–13% loss but status of most taxa is unclear. Isbell et al. (2022) had ~30% terrestrial invertebrates either threatened or extinct, similar to the ~30% rates in an IUCN’s “Redlist” of earthworms of Japan and NZ as compiled by the author in 2018.
While Prokaryotes may rapidly replace, Eukaryote extinctions are irrevocable. Thus time and resources on futile hunts for Life in Space or abysmal deep-sea biota while the soil ecosystem collapses beneath our feet seem like shameful, indefensible distractions.
3.4. Ocean Biodiversity &Biomass
No wholly marine fish nor reef coral is verified extinct in recent 250 years (recentlyextinctspecies.com/; vermecology.wordpress.com/2020/11/28/shed-in-the-sky/; vermecology.wordpress.com/2022/04/21/baka/; www.scientificamerican.com/article/smooth-handfish-extinction-marks-a-sad-milestone; nc.iucnredlist.org/redlist/content/attachment_files/20210909_Petition_Ruling_Smooth_Handfish.pdf; en.wikipedia.org/wiki/Smooth_handfish; https://news.mongabay.com/2021/03/corals-are-struggling-but-theyre-too-abundant-to-go-extinct-study-says/). Compared to Soil, Ocean is not at all biodiverse despite categorical claim such as from Ferrer et al. (2019): “the ocean.. hosts the largest population of microbes on Earth. More than 2 million eukaryotic and prokaryotic species are thought to thrive..” Clearly disproven as is reported above, Ocean has <0.01% while soil biodiversity alone is 1024 taxa. Ocean’s biomass at ~5 Gt C is <0.01% of Land’s >2,000 Gt C (Tabs. 1–2). Q.E.D.
PART 2 – Global Carbon Cycles
3.5. Gross Primary Production (GPP) and its Net (NPP) in Further Detail
Earth as a living entity has (most if not all) primary productivity due to chloroplast endosymbionts in plant cells, and respiration from mitochondria or soil microbes. Photosynthetic GPP CO2 carbon fixation is countered by Plant and Soil Respiration (PR + SR).
The most recent Global Carbon Budget (ESSD 2022: fig. 2) modified several soil values slightly, acknowledging an increase in terrestrial GPP to 130 Gt C/yr (with NPP about half at 65 Gt C/yr?). All their rates and values require revisionary review for latest soil and NPP data, not least to include consideration of gross errors for terrain omissions.
Using C and O isotopes, Welp, Keeling et al. (2011), Liang et al. (2017), and Laskar et al. (2019) gave CO2 carbon turnover times of 0.9–2.8 yrs (median ~1.8 yrs), mainly due to terrestrial activity. Welp et al. (2011) had 475–897 Gt C/yr and atmospheric C turnover in 0.9–1.7 yrs (mean ~1.3 yrs). They said: “This gross exchange flux can be used to estimate GPP by subtracting air–sea exchange” which is about ±80 Gt C/yr thus presumably 395–817 with mean ~600 Gt C/yr on land. With other adjustments, seemingly all 875 Gt C atmospheric CO2 is processed in around ~1.5 yrs. The fast response they plausibly accounted for by revising global GPP value upwards to 150–175 Gt C/yr for a GPP turnover of ~5 years.
In a summary of the status of global NPP estimations, Chapin & Eviner (2014) said: “NPP includes the new biomass produced by plants, the soluble organic compounds that diffuse or are secreted into the environment (root or phytoplankton exudation), the carbon transfers to microbes that are symbiotically associated with roots (e.g., mycorrhizae and nitrogen-fixing bacteria), and the volatile emissions that are lost from leaves to the atmosphere (Clark et al., 2001). Most field measurements of NPP document only the new plant biomass produced and therefore probably underestimate the true NPP by at least 30%.” Their table 1 showed only 30–70% of NPP in new plant biomass (median about 50%). Moreover, all ground surveys are based upon planimetric, flat biomes and thus fail to account for terrain. This may at least double the NPP estimates. Furthermore, Chapin & Eviner (2014) found boreal NPP to be especially underestimated, saying: “boreal forests, where NPP estimates are 75% greater than those of Saugier et al. (2001). Therefore, boreal NPP may be underestimated relative to other biomes.” Taking such factors into account, from a starting NPP of 70 Gt C/yr, the revised total may well be in the region of plus 50% to give ~105 Gt C/yr, doubled for terrain to ~210 Gt C/yr.
Continental NPP in Rodin et al. (1975) was >86 Gt C/yr, just 10% boreal; doubled is >172 Gt C/yr plus 21.5% greening effect in 50 yrs ups this to 210 Gt C/yr, as noted below. (Of note is that if boreal NPP at ~60 Gt C/yr is truly 10%, global total is ~600 Gt C/yr!).
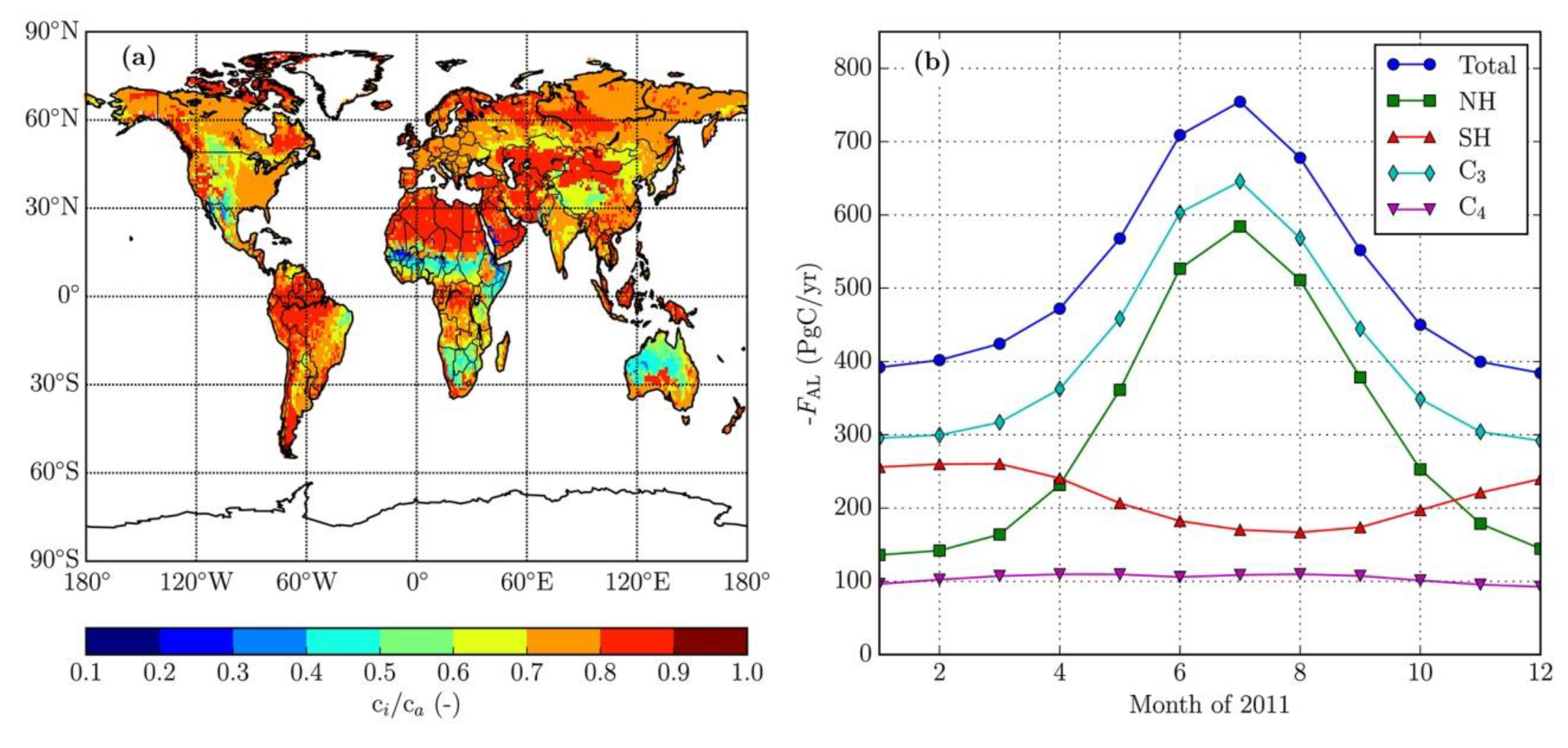
An undeniable CO2 summer flux at NOAA’s Point Barrow site is -40 Gt C from its catchment >35°N; Riach et al. (2002: figs. 2,4) show counter SR summer flux >30°N of (+80.3 ÷ 4 for half a year and half a hemisphere =) ~+20.1 Gt C to total -60.1 Gt C real summer NPP drawdown. This is supported by Bartsev et al. (2012: figs. 1–3) and by Basile et al. (2020: tab. 1; fig. 4). Also, if as Haverd et al. (2020) state: “land north of 35°N contributes less than 25% to global GPP”, then total global NPP may be (>60.1 x 4 =) >240.4 Gt C/yr, or tolerably above ~218 Gt C/yr NPP estimate (Blakemore 2018b, 2020c) that itself is 4 x prior “official” guesstimates of just around 55 Gt C/yr terrestrial NPP.
Koren et al. (2019: fig. 1) model 400–514 Gt C/yr leaf<->air flux and 111 Gt C/yr for soil respiration. They have a soil<->air flux of just 30 Gt C/yr while admitting: “
global soil invasion flux cover a wide range: from 30 PgC/year (Stern et al., 2001) to 450 PgC/year (Wingate et al., 2009)”. Their figure 4 has leaf<->air annual flux from 400 to 750 Gt C/yr (
Figure 15).
Remarkably, without giving explicit estimates of total global GPP nor NPP, Haverd et al. (2020: fig. 2a) seems to show a 2020 GPP model of ~143 GtC/yr (= NPP >71 GtC/yr?). Canadell et al. (2021: fig. 5.12) also had “Gross photosynthesis” or GPP of 142 Gt C/yr to give NPP about half this at ~71 Gt C/yr. Their studies support Campbell et al. (2017) who already showed a “large GPP growth during the twentieth century (31%)” with “measurement-based estimates of GPP are as large as 175 Pg C yr-1” (with NPP of 87 doubled to 174 Gt C/yr?). As several authors quote Koren et al. (2019), presumably they accept current GPP may be as high as 350 Gt C/yr (also giving ~175 Gt C/yr NPP?). Some prior NPP/GPP guesses are more or less wrong than others; Scurlock & Olsen (2002: tab. 3) showed not only that belowground NPP was about the same as aboveground NPP but, remarkably, also that few studies – perhaps half – considered roots. Raich et al. (2002) noted that a study by Keeling et al. had variabilities about twice theirs revealing large uncertainties in SOC and NPP, so much so that Scurlock et al. (2002) had NPP estimates 2–5 times higher than previously. This latter study median error of 3.5 times on current 60–70 = 210–245 Gt C/yr which fully embraces estimated NPP in Blakemore (2018b) of 218 Gt C/yr. Q.E.D.
3.5.1. NPP Assimilation is Countered by Soil Respiration and/or Decomposition (SR)
Because roots are so often ignored, perhaps SR gives a better proxy for global NPP. Bahn et al. (2010) had 98 Gt C/yr while Hashimoto et al. (2015) determined 91 Gt C/yr global SR increasing at a rate ~0.1 Gt C/y. Allocation was ~51 Gt C/yr from boreal and ~40 Gt C/yr from temperate or tropic zones that seems at odds with Haverd et al. (2020) claim of <25% boreal NPP and with some other NPP data presented herein. Sha et al. (2021) had SR of ~88 Gt C/yr with 22 Pg C/y, or almost a quarter, emitted from Asian soils. Other SR total CO2 release estimates range 88–111 Gt C/yr (Koren et al. 2019: fig. 1, Warner et al. 2019: tab. 1, Zhao et al. 2017, Hursh et al. 2017); all based upon planimetrically-flat land areas, when doubled for terrain, are up to 222 Gt C/yr balancing approximately 218 Gt C/yr NPP (Blakemore, 2018b). Thus – as is already stated and demonstrated above – soils may emit about twenty times more carbon than burning of fossil fuels does.
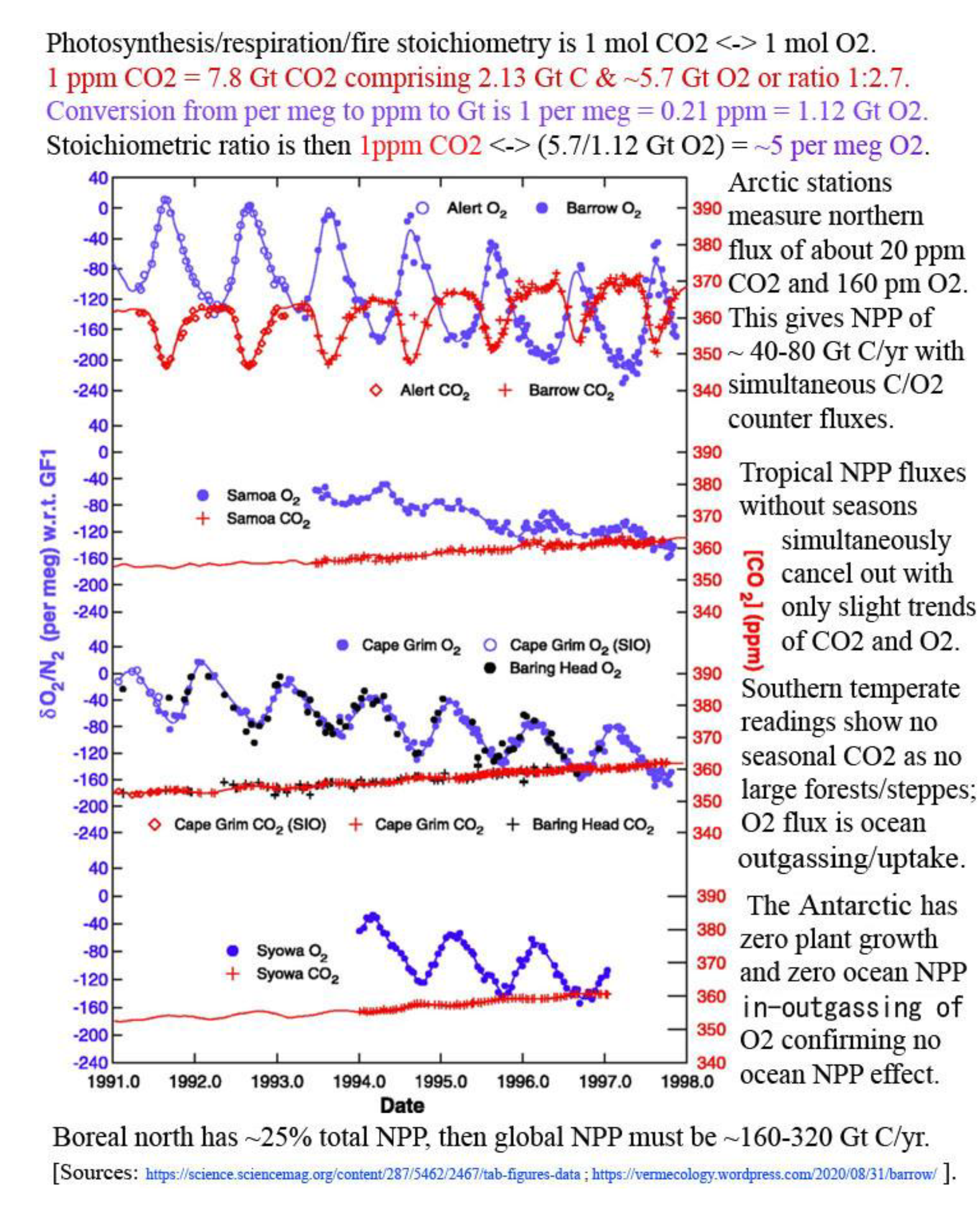
Further proof of neglected land NPP/SR is a report by Graven, Keeling et al. (2013) of extra 32–59% northern CO2 flux in just 50 years attributed to “growing season uptake increased by 40 to 60%, whereas dormant season release increased by only 20 to 50%”, this they imply from land change as they clearly state: “fossil fuel and biomass burning emissions make only minor contributions to the overall cycle” [just 1–6%]. The Ocean had no appreciable effect on carbon cycle fluxes [just 3–4%] as shown in their tab. S4. Moreover, their figs. 4 & S9 reveal neither their explanations nor CMIP5 models matched or could explain NPP/SR discrepancies observed neither at Barrow nor Moana Loa sites (due to ignored terrain?).
Graphical context of NPP CO
2 emissions with C Cycle drawdown are in
Figure 16.
3.5.2. Greening Effect Disproportionate to Accelerating Atmospheric CO2
Li et al. (2017: fig. 1a) determined that global greening increased NPP by 21.5% from 1960 to 2010. Similarly, Haverd et al. (2020) found a 30–47% increase in plant growth from 40–50% increase in CO2 with GPP estimated to have risen by +30% since 1900 or by +47% from pre-industrial levels with rising atmospheric CO2 concentration as the dominant driver. Comparatively, atmospheric CO2 increased ~50% from ~278 ppm in 1750 and 40% from 295.8 ppm in 1900 up to ~415 ppm today or by +119.5 ppm x 2.124 = +254 Gt C. This implies 30% or a 76.2 Gt C plant/soil carbon sink since 1900. Haverd et al. fig. 2a show terrestrial NPP increased to ~70 Gt C/yr. Rising land temperatures with upped soil activity must surely also play a part while water or nutrients may be limiting brakes. However, that vegetation NPP increase lags behind CO2 is likely due to the clearing of forests and erosion of topsoil ensuring it cannot keep pace with CO2 fertilization effects.
An increase in leaf-area-index (LAI) since 1981 was determined by Chen et al. (2019) with CO2 being greatest stimulant (+47%) and only a minor contribution from N deposition (+1%), climate being detrimental (-29%). A study by Keenan et al. (2021) plotting 12% increase for 17% extra CO2 from 1984–2020 was subsequently retracted seemingly due to critical uncertainties in their fig. 1. Earlier, Piao et al. (2020) concluded that CO2 fertilization is the main driver of global vegetation greening although warming is the major cause in boreal and Arctic biomes, having negative effects in the tropics. Zhu et al. (2016) showed warming affecting from 25–50% of vegetated lands from 1982 up to 2009 or 2014 with only 4% “browning”. Their global ecosystem models suggested that CO2 fertilization effects explained 70% of the observed greening trend, followed by nitrogen deposition (9%), climate change (8%), and land cover change (LCC) (4%). Nevertheless, a more extensive study from 1982–2017 reported only 40% greening and 14% browning indicating a disproportionately low increase of leaf area in recent years (Winkler et al. 2021a).
That CO2 continues to outpace plant drawdown indicates photosynthesis is unable to adequately recycle extra CO2, the reasons for which may include limitations of other resources such as water, vegetation loss especially by deforestation, and topsoil erosion inhibiting plant growth while also releasing yet more CO2 that exacerbates the problem.
3.5.3. Leaf-Area Index (LAI) as Backcheck on NPP
As a backcheck, a bio-mechanism to explain reasonableness of upping NPP values is Leaf-Area Index (LAI) which Asner et al. (2003) had as mean 4.5 m2/m2. Thus on a productive “flat” land basis of 12 Gha, the total leaf-area is 54 Gha. Doubled for coarse terrain is 108 Gha. However, as average leaf-area is at cm2 scale, refinement may require a redoubling to 216 Gha. Related to this, Field et al. (1998) had average NPP on land without permanent ice cover of 426 g C/m2/yr thus, with mean LAI of 4.5, gives a rough NPP/LAI ratio of 100 g C/yr/m2 leaf-area (= 0.01 g/cm2 or 1 t/ha). Thus 108 Gha leaf-area gives ~108 Gt C/yr and 216 leaf-area gives ~216 Gt C/yr for global NPP. Q.E.D.
Rather than just doubling, this fourfold terrain multiplication factor seems appropriate in suitable flat-Earth metrics (as indicated in Blakemore 2018b: tabs. 5–6), thus the doubled soil estimate values presented in the current work are possibly too moderate. Such guesstimates, however, are variable since Whitman et al. (1998) assumed LAI of 10, Fang et al. (2019) imply 2; Rodin et al. (1975: tab. 4) NPP equates to 525 g C/m2/yr. Returning to real-world mode, forest clearing erodes humic topsoil, reducing LAI and NPP.
3.6. Ocean NPP, Carbon & Oxygen Cycles in Context – See Appendix C
As for NPP, seemingly all ocean estimates (e.g., IPCC, ESSD, etc.) track back to Revelle & Suess (1957: tab. 2) based “in part after HUTCHINSON (1954)” that was “too uncertain to allow any definite conclusions”. Hutchinson (1954: 380) had NPP on land just 20 ± 5 Gt C/yr saying these figures were too low as they failed to account for tropical rainforests(!). Ocean NPP guess was five time greater at 126 ± 82 Gt C/yr he said was likely “an order of magnitude too high”. Indeed. These mystical figures (126 vs. 20 Gt C/yr, ocean vs. land) come directly from Riley (1944: 134), an obviously speculative and flawed work.
Rodin et al. (1975: tab. 1) refined Ocean NPP to 30 Gt C/yr and Continental NPP to 86 Gt C/yr. Woodwell et al. (1978: tab. 1) had marine NPP as 24.8 Gt C/yr and land just 52.8 Gt C/yr, whereas Siegenthaler & Sarmiento (1993) gave marine biological production of 10 Gt C/yr compared to terrestrial NPP that was five times greater at 50 Gt C/yr.
Compared to the current terrestrial NPP of ~220 Gt C/yr, there is little evidence for ocean NPP much above 10–30 Gt C/y supporting its total biomass of just ~3–6 Gt C. Reports are of NPP just 15–25 Gt C/yr and an Oceanic DOC pool of only 0.2 Gt C (e.g., https://eos.org/features/dissolved-organic-matter-in-the-ocean-carbon-cycle): “With this fast turnover, the pool’s contribution to carbon sequestration is inconsequential.”
While Rodin et al. (1975) have ocean phytomass of just 0.085 Gt C, presumably fish etc. provide extra to total ~3–5 Gt C marine biomass. Of this, ~100 million tonnes wet weight is captured or extracted each year (www.fao.org/3/cc0461en/online/sofia/2022/world-fisheries-aquaculture-production.html). Supporting data is presented here - ourworldindata.org/fish-and-overfishing. Nevertheless, 100 million tonnes wet catch is ~30 million tonnes dry and 15 million tonnes of carbon, or 0.015 Gt C/yr; only <0.5% of Oceans’ total biotic stock or just 0.1% of its NPP.
Comparative human appropriation of NPP (HANPP) on land is 1,000 times greater at ~15 Gt C/yr from Krausmann et al. (2013) who include fire and LUC, or when compared to Alexander et al. (2017) who have ~13.9 for food alone with 8.0 and 5.9 Gt C/yr for croplands and grassland, respectively. This represents about ~23% of total terrestrial NPP when taken as just 60 Gt C/yr, or now just 6% for Land’s total NPP of ~220 Gt C/yr.
Moreover, sea<->air gas exchanges are passive and instantaneous as governed by Henry’s Law. Hence, Ocean’s overstated importance to global CO
2 or O
2 cycles are in fact relatively minor, as Koren et al. (2019) say: “
the gross ocean fluxes largely cancel out” (just ∼±3 Gt C/yr globally). Unlike from Land, Piao et al. (2019: fig. 9) show negligible, or negative, seasonal/annual Ocean contribution on total global carbon fluxes. Carbon CO
2 fluxes in Fig. 16 above are extended in supporting data presented below (
Figure 17).
Global O2 levels give insight into SOC loss as stoichiometric photosynthesis:decomposition molar ratio (on land!) of O2:CO2 (also O2:C) is 1:1 and per meg O2 loss to ppm CO2 gain is confirmed as about 10:1. Atmospheric O2 declined -600 meg in 30 years from 1990–2020 (https://www.oxygenlevels.org/) or -20 meg/yr; at the same time, CO2 accumulation from 1990–2020 was 354.5 to 414.5 ppm (+60 ppm or 2 ppm/yr with about +4.2 Gt C/yr). This net ~4 Gt C/yr is both by burning of fossil fuels, which is mostly accounted for, or by loss of SOC, which is not. Aside from fossil fuels and fires, ~8.5–9.2 Gt C/yr is due to soil degradation loss, much of which is taken up by terrestrial greening.
3.6.1. Myth of Ocean Oxygen (or the O-O Fallacy)
False and misleading claims that Ocean supplies 50–90% of oxygen needed to breathe each year are countered as its outgassing flux of just ~0.00015%/yr compared to ten times as much at 0.0014%/yr from land’s NPP. Manning & Keeling (2006) had a lower annual Ocean outgassing contribution to the atmosphere’s 21% oxygen (37,000 Pmol O
2 or about 1.18 million Gt) of 0.048 Pmol O
2/yr or just 0.0001%/yr. In factual reality, there is a small net loss of atmospheric oxygen due mainly to burning and SOC loss (
Figure 18).
3.7. Acidification and Plastication in Context
Soil infertility and susceptibility to erosion are both intensified by increasing acidity and toxicity. NOAA and many other marine institutions distract with claims that ocean “acidification” is a problem as it has basically gone from pH 8.2 to 8.1 (or -0.1) over the last few centuries that they say is a 25–30% decrease due to the logarithmic scale. However, as only ~0.3% of human food comes from the oceans that support less than 0.001% of global biodiversity it is not so critical. Despite being almost always ignored, soil acidity is a much greater problem at orders of magnitude higher occurring much more rapidly at a rate about 26 times higher than in the oceans and only in the last few decades, mainly due to agrichemical intensification. An example, from Tian & Niu (2015): “We found that N addition significantly reduced soil pH by 0.26 on average globally. However, the responses of soil pH varied with ecosystem types, N addition rate, N fertilization forms, and experimental durations. Soil pH decreased most in grassland, whereas boreal forest was not observed a decrease to N addition in soil acidification”. Soil acidity disrupts or destroys soil fauna and plants including trees or saplings (https://en.wikipedia.org/wiki/Soil_acidification) and releases toxic Aluminium/Aluminum (Al) that also stunts roots. Especially conifer forests that are already associated with more acidic podzols (or podsols) have a compacted humus layer, known as mor, microbially dominated by fungi. Nevertheless, there is an urget need “for stringent measures that reduce sulfur and nitrogen emissions so as to maintain ecosystem structure and function” (Yang et al. 2015). Acid rain, long recognized as a major challenge, is due to many natural or human factors but mainly agrichemicals. As well as Nitrates or Sulphates, increasing CO2 forms Carbonic acid adding to acid rains (en.wikipedia.org/wiki/Acid_rain), seemingly as does Formic acid (Stavrakou et al. 2011).
On average, soil pH has reportedly decreased 0.24–0.26 pH points globally – a 100% change (Tian & Niu 2015, Blakemore 2018a, Meng et al. 2019), more so under intensive agricultural situations by up to two pH units or by 10,000% in just a few decades (Fenn et al. 2006, Delang 2018, Guo et al. 2018). Thus ocean acidification of <30% in two centuries is quite trivially irrelevant (vermecology.wordpress.com/2021/02/02/ocean-arsidity/). Meng et al. (2019: fig. 5) showed acid soils release Al or Fe and decrease roots by 19% and soil bacteria by 16.4%. Reports as early as 1920s already showed decline of soil fauna (e.g., insects, microbes, “worms”) and complete depletion in acid soils (pH <3.0) at Rothamsted after synthetic fertilizer introduction (Morris 1922, 1927; Blakemore 2018a).
Popular or political alarmism for plastic pollution in seas are an order of magnitude greater in soils, being 23–32 times worst (unep.org/news-and-stories/story/plastic-planet-how-tiny-plastic-particles-are-polluting-our-soil; ieep.eu/news/isqaper-exploring-plastic-pollution-in-soil). Microplastics damage soil organisms, in particular earthworms (e.g. Cui et al. 2022, Tibbett et al. 2020). The latter paper summarizes: “The lack of research effort in key areas of high priority in the threats to soil biodiversity are a concerning finding and requires some consideration and debate in the research community.” Such debate is mostly heretofore lacking. A requisite now is honest, fact-based, context-triage to resolve pollution issues in order of importance and urgency.
PART 3 – SOC Loss
3.8. Land Use Change (LUC) Contribution with Fossil Fuels (FF) to Atmospheric CO2
An initial report by IPCC (2000) was updated in February 2022 with their Special Report on Climate Change & Land (IPCC 2022) part of a larger 3,949-page Report. Therein they define Land Use Change (LUC) as: “The change from one land use category to another” implying vegetation change (ambiguity is if this is above-ground only or also including roots and other soil components?). Moreover, soil erosion and SOC loss are often – but not always – treated separately, thus the two entities: LUC and SOC loss, mutually interlink and intergrade in surveys, studies or reports such as the current one.
On 11
th November 2022, following latest Global Carbon Budget Report (ESSD 2022: fig. 2;
https://doi.org/10.5194/essd-14-4811-2022), new LUC compilations by OWiD (
ourworldindata.org/co2-data-update-2022;
ourworldindata.org/explorers/co2) used Global Carbon Project data (
www.globalcarbonproject.org/carbonbudget/22/data.htm). Consensus cumulative emission values from 1850 to 2022 converge on ~205 ± 60 Gt C due to LUC and ~465 ± 25 Gt C for FF, with increase of atmospheric CO
2 from ~285 ppm in 1850 to 415 ppm currently to total +275 Gt C (
Table 3).
* Speculative data (Kaplan et al. 2010: tab. 4, IPCC 2013: 484, Erb et al. 2018: tab. 3); cf. Gasser et al. (2020) that concluded LUC loss of 206 ± 57 Gt C in the period 1750–2018. ** LUC of 410 in 170 yrs is 2.4 Gt C/yr; recent loss rate of 193 in 70 yrs is 2.8 Gt C/yr. *** Total natural and human LUC throughout Holocene of 682 + 410 is 1,094 Gt C. A section below on ignored roots in Land Carbon “Debt” has a similar LUC of ~1,078 Gt C but what proportion of this (20–50%?) directly volatilizes to CO2 is not entirely certain.
While FF values are easily accounted (now ~9.6 Gt C/yr), LUC is more complex. Following Blakemore (2018b), LUC calculations based upon planimetrically-flat biomes are at least doubled for terrain. Rather than 325 Gt SOC loss since 1850 as suggested in the Introduction (and Appendix A), a new LUC value is thus (205 x 2 =) 410 Gt C that, when added to corresponding FF estimate of 465 Gt C, is 875 Gt C total emissions (+5 Gt C/yr). This value is >30% above ESSD’s estimated 670 Gt C total emissions (+4 Gt C/yr). That atmospheric CO
2 remains unchanged at ~+275 Gt C may be explained by an accompanying +30% increase in NPP drawdown due to a CO
2 fertilizing effect on warmer land (as per Haverd et al. 2020). In addition to this, it is speculated that LUC has accelerated and the global vegetation turnover times (τ) has halved from a potential biomass τ of 13.7 yrs compared to 7.1 yrs in the current era (Erb et al. 2016). The many authors on IPCC or ESSD Global Carbon Report are invited to comment on or to correct
Table 3 data.
A recent paper by Wang et al. (2022) modeled global SOC loss to 2 m depth under 1°C air warming of between 620±130 to 1,551±293 Gt (similarly doubled for terrain?). Since temperature is already risen 1°C from 1850s, such loss of presumably ancient deep SOC may have already occurred. Potential of such added SOC carbon to increase atmospheric CO2 considerably, both warming and greening the planet, cannot be ignored.
3.9. Topsoil Erosion Rates (Dry Mass orSOC Loss) and SOC Percentage (SOC%)
Various ‘flat-Earth’ erosion rates range 20–200 Gt dry/yr total topsoil loss with 1.4–7.4 Gt C/yr SOC loss, doubled for terrain these are around 3–15 Gt C/yr (Appendix A). Thus, Lal’s (2006: fig. 3.2) revised estimate in
Figure 1 of 8.0–12.2 Gt C/yr seems a reasonably reliable approximation. Erosional SOC loss estimates then fall within a range of between 3–15 Gt SOC/yr (with median value ~9.0 Gt C/yr) (
Table 4).
* Buringh (1984) reasoned mean 4.6 Gt C/yr, doubled for terrain is about 9.2 Gt C/yr. ** Median SOC erosion range between 3–15 Gt C/yr is also ~9 Gt SOC/yr. Mean SOC% (of 3.0, >0.7, 3.6%) is >2.4% close to an assumed mean value of 2.5% in the Methods section.
How do LUC carbon emissions >1850 compare or comply with current soil erosion rates?
Table 3 mean LUC from >1850 or from >1950 of 2.4 or 2.8 Gt SOC/yr are at lowest of range of those in the last column of
Table 4. This is because not all SOC loss is as CO
2.
Compared to Lal (2006: fig. 3.2) (
Figure 1), Lal (2006: tab. 3.2) showed global sediment and SOC erosion as 200 Gt/yr and 1.4 Gt SOC/yr that, despite being doubled for terrain to 400 and 2.8, respectively, give an unrealistically low SOC of just 0.7%. FAO (2015a: 1-1
www.fao.org/3/a-i5199e.pdf; 2015b:
www.fao.org/news/story/en/item/357059/icode/) had global topsoil erosion ranges from 20 to 200 Gt/yr but argue that reasonable figures are ~25 Gt/yr by water, another 5 Gt/yr for tillage and 2–3 Gt for aeolian dust to total ~33 Gt/yr. A similar water rate by Lal (
2022) of 36.6 Gt/yr in the absence of reservoir trapping emits ~1.1 Gt C/yr (SOC 3%). He estimated total amount of SOC displaced by sheet erosion from its source was 1.32 ± 0.20 Gt C/yr. Earlier, Lal (
2020) had: “
The global magnitude of SOC erosion may be 1.3 Pg C/yr. by water and 1.0 Pg C/yr. by wind erosion...” (total 2.3 Gt SOC/yr). Thus, current water erosion estimates by Lal (2020, 2022) are of dry topsoil loss of 36.6 Gt/yr (from “
Walling, 2008”), although Pimentel & Burgess (2013) had twice this total soil erosion at 75 Gt/yr (x 2 = 150), and Blakemore (2020c) had higher figures of 75.4–377 Gt/yr based upon reported erosion loss and average soil SOC%. Nevertheless, erosion losses are much higher than previous estimates, and a reasonable terrain-based rate is 150–400 (median 275) Gt/yr of dry soil.
Compared to Lal (2006: fig. 3.2) (
Figure 1), Lal (2006: tab. 3.2) showed global sediment and SOC erosion as 200 Gt/yr and 1.4 Gt SOC/yr that, despite being doubled for terrain to 400 and 2.8, respectively, give an unrealistically low SOC of just 0.7%. FAO (2015a: 1-1
www.fao.org/3/a-i5199e.pdf; 2015b:
www.fao.org/news/story/en/item/357059/icode/) had global topsoil erosion ranges from 20 to 200 Gt/yr but argue that reasonable figures are ~25 Gt/yr by water, another 5 Gt/yr for tillage and 2–3 Gt for aeolian dust to total ~33 Gt/yr. A similar water rate by Lal (
2022) of 36.6 Gt/yr in the absence of reservoir trapping emits ~1.1 Gt C/yr (SOC 3%). He estimated total amount of SOC displaced by sheet erosion from its source was 1.32 ± 0.20 Gt C/yr. Earlier, Lal (
2020) had: “
The global magnitude of SOC erosion may be 1.3 Pg C/yr. by water and 1.0 Pg C/yr. by wind erosion...” (total 2.3 Gt SOC/yr). Thus, current water erosion estimates by Lal (2020, 2022) are of dry topsoil loss of 36.6 Gt/yr (from “
Walling, 2008”), although Pimentel & Burgess (2013) had twice this total soil erosion at 75 Gt/yr (x 2 = 150), and Blakemore (2020c) had higher figures of 75.4–377 Gt/yr based upon reported erosion loss and average soil SOC%. Nevertheless, erosion losses are much higher than previous estimates, and a reasonable terrain-based rate is 150–400 (median 275) Gt/yr of dry soil.
A backcheck is median/mean value of % SOC loss from
Table 4 (range 0.7–3.6%) of 2.4% is near the assumed average of around 2.5%. Conversely, a 3–15 Gt SOC/yr range, if comprising 2.5% mean topsoil SOC, rather implies 120–600 Gt/yr loss compared to “official” erosion range 75–200 Gt/yr (doubled for terrain to 150–400). Whereas 75 Gt/yr topsoil loss is 2,000 dry tonnes per second (t/s). If 3–15 Gt C is truly lost with mean 2.5% SOC this represents 120–600 Gt/yr, with an equivalent loss rate of 3,750–20,000 t/s. This upper value of 20,000 tonnes of dry soil eroded per second (as given in Abstract) is an extremely high rate of erosion. However, the lower rates should also be of great concern.
Land’s rainfall at about 100,000 Gt/yr supports feasibility of high soil erosion rates.
Clear indication is that loss of SOC stock and terrestrial carbon cycles are as important, or more so, as contributory factors to atmospheric CO2 accumulation than are FF emissions alone. Nevertheless, as Lal (2021) noted: “The GCB [ESSD’s Global Carbon Budget] is strongly affected by accelerated erosion... However, the C emitted by erosion is not accounted for in the GCB.” Such oversight requires correction in their models and totals.
3.10. Reconciling Land Use Change (LUC) and SOC Loss with FF Emission Since Industrial Age
Land use change is often attributed to clearing of forests with erosion of soils. The latest ESSD (2022) report admits: “Uncertainties in current and historical carbon stocks in soils and vegetation also add uncertainty in the ELUC estimates. Unless a major effort to resolve these issues is made, little progress is expected in the resolution of ELUC.” The establishment of a directed Soil Research Institute, as is advocated herein, would help resolve such issues.
ESSD (2022: tab. 4 and
meta.icos-cp.eu/objects/IuLZ5Dkg7UoiSt_ZLskXbwGw) LUC budgets are certainly on a planimetric basis as their sources, e.g. Hansis et al. (2015) say: “
the net LULCC flux can be simply derived from information on carbon stocks of each land use state and the change in area”. Areas given are such as Li et al. (2018) , for example, having: “
global areas of forest, cropland and grassland PFTs from ESA as 30.4, 19.3 and 35.7 million km2” similar to unrealistic, mirror-flat areas as shown in Blakemore (2018b: fig. 4) (cf.
Figure 1).
In
Table 3, the “official” anthropogenic emissions from 1850 of LUC at 205 Gt C and FF at 465 Gt C give cumulative total 670 Gt with about half (670/276 = 41%) CO
2 accumulated. Yet, if terrain doubles estimated values of soil/land losses, total emissions would be raised and contributions from SOC/LUC or fossil fuels become commensurate.
In
Table 4 a summary of current rates of SOC loss requires remedy beyond the atmospheric CO
2 issues. Rather than just reduce FF emissions, a question must be how much of the excess ~276 Gt C CO
2 can be restored or sequestered via organic farming and pasture or forest regrowth? If soil erosion is reduced to offset annual atmospheric accumulation (of +5 Gt C/yr) then, with modest 2 Gt SOC/yr restored on farmland and forests, total drawdown of ~300 Gt C would take ~150 years. Atmospheric drawdown of 300 Gt C is equivalent to ~1 ppm/yr consistent with Lal’s (2009) argument for 106 Gt C soil sequestration to give a reduction rate of 50 ppm in 50 years (also ~1 ppm/yr). Such considerations of SOC losses and reasonable restoration rates are further assessed below.
3.11. Land Carbon “Debt” in Greater Detail
Erb et al. (2018) had found unexpectedly large impacts of forest management and grazing on carbon stocks in vegetation that would have potentially stored 916 Gt C rather than current (flat) 450 Gt C. A remarkable summary by Wuepper et al. (2021) had: “The above-ground biomass would naturally be 871 Gt C, but currently, it is only is 601 Gt C. This means our global above-ground carbon debt is 270 Gt C. Below-ground, naturally, there would be 899 Gt C, but currently, there are only 863 Gt C, which means our global below-ground carbon debt is 36 Gt C.” Apparently their “above-ground” refers mainly to trees, and “below-ground” is soil carbon debt of 12,000 years of human land use (for just upper 30 cm) based upon Sandeman et al. (2017) data. They seemingly ignore proper inclusion of roots (as noted above under Roots) or other biota. These losses they attributed to soil erosion and a “Land degradation debt”. They add: “In addition, our analysis suggests that global land use has so far decreased global tree cover by 30%, carbon stored in biomass by 20% (average for above- and below-ground carbon), and increased soil erosion almost fourfold, suggesting that our global soil erosion debt is especially large and deserving of special attention.” Unfortunately, their soil erosion debt of just 36 Gt C is obviously an underestimation when “official” global SOC erosion rate is in the order of 1–6 Gt C/yr, probably for the last few centuries. These data, and ESDD’s most recent values or rates, therefore require stark revision.
Regarding such “debt”, Elhacham et al. (2020) estimated since the Neolithic agricultural revolution, plants were roughly halved from 2,000 Gt (dry-mass) down to a current value of 1,000 Gt or a debt of about -500 Gt C. Biomass of (mainly soil) Bacteria and Archaea they found decreased from 80 Gt C to 30 Gt C or a “debt” of -50 Gt C only from 1900–1990. Falsely claiming “soil carbon is not living biomass and thus is not included”(!), they surprisingly excluded SOC from their study entirely. Combining all their biotic data sets, their above- and below-ground “carbon debts” total -586 Gt C that, doubled for terrain, are -1,172 Gt C. Should current SOC losses – as determined herein – be added to total plant loss, then total land carbon debt is truly massive. Indeed this was recently determined by Georgiou et al. (2022: tab. S1) having a global soil deficit or debt of -543 Gt SOC.
Georgiou et al. (2022) found mineral soils – those mostly used in agriculture, excluding tundra, peatlands, or deserts – undersaturated by 21–42% of their carbon capacity. Total deficit in their table SI is 433–543 Gt SOC (with or without tundra). Cropland, grazing and forestry deficits were 104, 253 and 50 Gt SOC, respectively. Cropland change of ±104 Gt C theoretically alters (adds or subtracts) atmospheric CO2 by ~50 ppm.
With regards to the inexplicable omission of roots from most soil calculations, a new global value of 916 Gt C root stock allows re-evaluation of Wuepper et al. (2021) (
Figure 19). Their “
above-ground” 871 Gt -> 601 Gt debt is a reduction of -31% and, to return from 601 Gt -> 871 Gt, would require +45% above-ground vegetation increase. This implies the current 916 Gt C in all roots, if with a similar loss/gain ratio, would have initially totaled 1,330 Gt C. Thus, a corresponding root debt seemingly of ~414 Gt C is missed. Adding other doubled debts gives ~1,078 Gt C LUC that – as posited herein – is far greater than FF emissions (~465 Gt C) or LUC (410 Gt C) given in
Table 3. Remarkably, this LUC loss value almost exactly matches Buringh (1984) LUC loss at 537, here doubled to 1,074 Gt C.
3.12. Fire Emissions Often Neglected from Models
Just as soil erosion is often downplayed, so too are natural or human-set fires e.g., Canadell et al. (2021) report: “
Wildfire is included in some.. models”. Wildfires officially emit ~2 Gt C/yr (e.g. Zheng et al. 2021) with about a quarter from tropical deforestation plus peatland incineration; in 2021, global wildfires alone supposedly caused an output total of 1.8 Gt C. Yet previously, IPCC WGI-TAR1 (
2001: fig. 3–1) had 4 Gt C/yr from “
combustion” that, when doubled for neglected terrain, is 8 Gt C/yr (cf.
Figure 5 and
Figure 6). Their source was Scholes & Andreae (2000: tab. 2) who had African savanna fires at ~1 Gt C/yr and “
Biomass burning” or “
All human sources” at 13.5 or 33.7 Gt CO
2/yr (= 3.7 or 9.1 Gt C/yr). Including biofuel, crop stubble, and home garden or kitchen burning (rather than composting) contribution of fire to CO
2 is non-trivial (e.g.,
gfmc.online/wp-content/uploads/18-IFFN-31-Emissions-2.pdf tabs. 1–2). For human-lit fire, Andreae (2019: tabs. 1–2) had ~4.4 Gt C/yr but he noted FLAMBE model of 8.75 Gt dry matter burnt in tropical forests alone (= 4.38 Gt C). Andreae & Merlet (2001: tab. 2) had 1.33 Gt/yr dm (0.65 Gt C, x 6.6 less), suggesting a higher (4.4 x 6.6 =) 29 Gt C/yr total?
PART 4 – SOC Restoration
3.13. Studies on SOC Loss Over Decades or Centuries Point to Solutions in SOC Restoration
A fundamental feature of a healthy soil is undeniably its soil organic carbon (SOC) status. Intensive agrichemical farming is known to deplete or “mine” SOC. Three main sets of reference data are available: Heritage reports from early soil surveyors such as Count von Strzelecki in Australia; data from long-term experiments (LTEs) plots or fields that have preserved samples or recorded changes in SOC with time; and contemporary organic farms that act as proxies for original optima status compared to their conventional chemical neighbours. Re-attaining humic SOC levels may provide a safe way to reverse the harm bad Agriculture has had on soils over prehistoric to more recent times.
3.13.1. Count Strzelecki in Australia in 1839–1843
Between 1839 and 1843, soil carbon samples were collected from colonies of South-East Australia (prior to 1851, the state of Victoria was part of New South Wales, NSW) and Tasmania by Polish explorer and geologist, Sir Paul Edmund [Count] Strzelecki. It is perhaps significant that 1839 was also when the longest of long-term experiments commenced at Rothamsted in the UK to determine effects of different commercial fertilizers on crops and soils, these reference samples being consistently collected and contentiously stored since then with support from central Governments funds.
Strzelecki sent soil samples to the UK for analysis. According to Jones (2009), Strzelecki (1845) reported ten farm or paddock soils in a high productivity group with soil organic matter (SOM) levels 11–37.75% (average 20% or 10% SOC) and ten soils in a low productivity group with SOM 2.2–5.0% (average 3.72% or 1.85% SOC). Strzelecki’s original data are reanalyzed and corrected slightly to allow for differing soil moisture contents to now show overall mean for NSW (and partly Victoria) of 4.1% SOC and for Tasmania 4.5% SOC. These are substantially above contemporary values showing SOC decline of between 40–50% (cf. Appendix B, Supplementary Strzelecki Excel file).
This is consistent with Dr Bill Coching (2018: fig. 3) finding Tasmanian SOC decline from mean of 5% to 2.5% in ~40 years of cultivation (-50%). Dr C. Jones’ summary: (web.archive.org/web/20200315053953/http://www.farminstitute.org.au/_literature_68254/AGE2010_Paper_Dr_Christine_Jones) was that “Strzelecki’s data indicate that organic matter levels in the early settlement period were around five to ten times higher than in many soils today”; and Jones (2009) report of normal SOC in Victoria of 0.9–5.8 % with median ~3% compared to SOC of 1.1–18.8 with median ~9% found by Strzelecki in NSW (partly Victoria). As Jones (2009) cogently remarks: "On average, 12 tonnes of topsoil are eroded for every tonne of wheat currently produced in Australia. Greater losses are experienced on more fragile soils. For example, over 200 tonnes of topsoil are eroded for each tonne of wheat produced in some parts of the Wimmera region in western Victoria. No civilisation can survive the physical destruction of its primary resource base - the soil." Such information indicates a serious issue.
3.13.2. Long-Term Experimental (LTE) Fertilizer Results for SOC, Yield, Biodiversity, Etc.
A remedy for SOC loss may be found in restoration of 100% organic farming. Therefore, accounts of SOC values of various LTEs are analyzed, not just for changes from original starting levels, where available, but also comparison of organic treatments if taken as preserving the de facto starting values since they best represent prevailing management prior to synthetic fertilizer usage (Blakemore 2018a). Trials often use Farm-Yard Manure (FYM), a type of coarse compost, or other less-than-optimal practices.
Organic farming is properly concerned with maintenance of soil health and this too is the foundation of Permaculture. Applying the principals and practices of Permaculture (Mollison 1988) allows much flexibility and a transition, where appropriate, from high-maintenance, annual monoculture to self-supporting perennials such as fruits, nuts, or vines; combining with Yeoman’s (1954, en.wikipedia.org/wiki/Keyline_design) Key-Line Planning for rainwater management, an issue in both drought and flood areas.
An assertion often made is that synthetic N fertilization maintains or increases SOC by enhancing production of crop residues, yet the opposite effect was realized in early days (e.g., Howard 1945, Balfour 1943). This is consistent with evidence that adding N enhances microbial decomposition of plant residues (e.g., Mulvaney et al. 2009) hence actually reducing SOC. Depletion of soil carbon exacerbated by adding synthetic Nitrogen deliberately or vicariously via global rainfall pollution. A meta-analysis on 257 studies on effect of soil N addition on soil had substantial stimulation of soil respiration (16%) and an enhancement in soil C mineralization (6%) within agricultural ecosystems (Lu et al. 2011); however, variable results were claimed from pot and field trials in Canada (Gagnon et al. 2016). Such information may be verified with reference to LTE results.
The majority of long-term field experiments were founded in Europe or North America: e.g., at Rothamsted or Woburn (UK), Askov (Denmark), Grignon (France), Bad Lauchstädt (Germany), and Morrow plots or Sanborn Field (USA). These and other sites are listed online at - https://glten.org/. Summary of a over a dozen LTE trials (vermecology.files.wordpress.com/2022/09/no-nox-2.pdf) showed overwhelmingly positive results, with many essential benefits of organics (such as conserving earthworms, microbes, SOC carbon sequestration, etc.). Reports show organic FYM (Farm-Yard Manure) often excelled over synthetic N-P-K fertilizers despite, in most cases, neither optimal organic practices such as crop or fallow rotations, nor good composts being used.
A field-study, meta-analysis by Blakemore (2018a) found yields +17.8% higher, SOC/SOM humus depleted -56.8%, soil moisture -22.3%, and earthworms reduced -83.2% from optima in side-by-side organic vs. N-P-K farm trials. Microbes were significantly depleted by chemical farming in those soils tested (by -50%). An assumption was that the organically (FYM or compost) managed fields represented starting level status.
Kahn et al. (2007: tab. 3) tabulated about 50 long-term N fertilization trials from around the World, all with rates of carbon loss at the final sample compared to the first reduced by up to -39%. Only three positive increased in carbon sequestration were noted in a report by Buyanovsky & Wagner (1998) from Sanborn Field. Sanborn Field at the University of Missouri-Columbia, was established in 1888 with rotation and manure treatments on 39 plots. After a century, Buyanovsky & Wagner (1998: tab. 4, fig. 2) show continuous manure treatments conserved SOC nearer original levels unlike other plots.
Grace et al. (1995) reported on a long-term (commenced 1925) rotation trial at Waite in South Australia that for the 11 rotations, had SOC in the top 10 cm decline from 2.75% in 1925 to a mean of 1.56% in 1993 (or by -43.3% in about 70 years). One plot, which had reverted to permanent pasture in 1950, showed the smallest decline with an SOC content of 2.46% in 1993 (-10%). Such starting SOC values are somewhat lower than Strzelecki’s.
Data on 120 yrs of yields and soils at Palace Leas, UK also noted acidification by ~2 pH units (vermecology.wordpress.com/2022/05/04/worms-in-the-palace/). A summary by Standen (1984: tabs. 1, 6) had SOC 5.3% in FYM vs. 3.7% in N-P-K plots (or -30.2%).
LTE values of -30.2–56.8% SOC decline support the thesis that intensive agrichemical farming depletes crop or pasture soils by about half of their organic humus potentials.
3.13.3. Imperative to Reduce Synthetic Nitrogen Fertilizers
Outside of a necessity for SOC restoration to revive soil health and natural fertility, there is a corresponding drive to reduce artificial fertilizers that are conclusively shown as deleterious to soil and human health, many foods being depleted in essential minerals and nutrients in the last 60 years (Thomas 2007: tab. 9). As further evidence for this, Mayer et al. (2022) recently reviewed Organic food with higher mineral nutrients and dry matter (but less moisture that adds to supposed yield hikes). They concluded: “For a range of reasons, agroecological farming should be supported in the context the global climate emergency and loss of biodiversity. The limited evidence that compares nutritional value of organic versus conventionally grown F&Vs [Fruit & Veggies] adds an extra reason to switch from industrial to agroecological farming.” Fan et al. (2008) showed Rothamsted’s Broadbalk wheat depleted in minerals over 160 years except in organic FYM plots that were similarly depleted from 1960s when N-P-K and herbicide (MCPA) were applied (although the authors fail to consider these as contributory factors, cf. Blakemore 2018a: figs. 1, 8) along with introduction of agrichemical dependent “Green Revolution” dwarf varieties.
Global crop yields increased 200% or 3 x since intensification in 1960s, yet 800% or 9 x extra synthetic N was used with most lost as environmental contaminant (Figs. 20–21).
As Mulvaney et al. (2009) confirmed: “Cereal production that now sustains a world population of more than 6.5 billion has tripled during the past 40 yr, concurrent with an increase from 12 to 104 Tg yr−1 of synthetic N applied largely in ammoniacal fertilizers”. This synthetic N total is less than natural stock cycles, as already alluded to for Earthworms (with 30 x as much N), biofilms (processing >100 Tg N/yr), both trivial to microbial biomass with 200 Gt C and 48 Gt N (= 48,000 Tg Nitrogen). Demise of soil biota may deplete this nutrient, but re-evaluating the true benefits of synthetic N fertilizer is yet required. Using Reiners (1974) C:N ratio of 12, an NPP/SR of 220 Gt C/yr naturally recycles at least 18 Gt N/yr.
Cereal yields are not the only metric as other crops and smallholder production contribute, previously estimated as 70–80% recently revised to ~30% (Ricciadi et al. 2018, 2021). FAO (2013: 130 - fao.org/docrep/018/i3107e/i3107e.PDF) say: “Of the approximately 2.3 billion tonnes of cereals currently produced, roughly 1 billion tonnes is destined for food use, 750 million tonnes is employed as animal feed, and the remaining 500 million tonnes is processed for industrial use, used as seed or wasted.” An extravagance is to feed grain to grazing stock.
Several LTEs (above) and other reports noted organic yields as high – or higher – than adjacent conventional. Transition to small, local farms rather than trending to larger broadacre arable gives more variety and higher production. Allotments or home gardens can yield two to ten times as much food per unit area (Watson 2015) using less toxic chemicals for their produce.
Figure 21 again shows yields correlate more with area and volume of irrigation over the same time period than to use of fertilizers or toxic biocides.
Nitrates in water are not the sole problem. Since the Montreal Protocol implementation banned CFCs, synthetic N fertilizers again pose the greatest threat to our life-protecting Ozone (Forster et al. 2020). Mohr (2021) shows it was known since the early 1970s, e.g., Crutzen (1970), that: “Nitrous oxide emitted from the application of fertilizer, among other processes, breaks down in the stratosphere and represents a source of ozone-depleting nitrogen oxides.” (Also see summary - vermecology.wordpress.com/2022/09/01/no-NO/).
3.13.4. Toxic Agrichemical Biocides
Amongst many as yet not fully blanket-banned agrichemicals are formulation of Atrazine by ChemChina/Syngenta or Glyphosate by Bayer/Monsanto used as herbicides or crop drying agents. Glyphosate is especially counterproductive since it is patented as an anti-microbial (Patent No. US7771736B2). Not limited to Enterobacteriaceae essential to our digestive health, it is also toxic to (N-fixing) soil bacteria (Santos & Flores 1995, Van Bruggen et al. 2018), fungi (Vazquet et al. 2020), algae, and other organisms (Singh et al. 2020). Glyphosate was known to affect essential eukaryotic mitochondria function since 1970s (Olorunsogo et. al. 1979, Peixoto 2005, Myers et al. 2016, van Bruggen et al. 2021, Strilbyska et al. 2022, Mesnage et al. 2022). How much this destruction of soil biota adds to soil CO2 or NOx emissions is unclear as this subject seems as yet uninvestigated.
Such chemicals pollute our soil, air, water, food, and bodies being linked to infertility and severe human or animal illnesses, including cancer. Formulations are toxic to soil fungi in ultra-low doses (Nicolas et al. 2016) and play a non-trivial role in emergence of antibiotic resistant bacteria (Raoult et al. 2021, Liao et al. 2021), although this is a common characteristic amongst competing soil microbes, especially fungi (Bahram et al. 2018). Deleterious effects of glyphosate on earthworms are well reported (Gaupp-Berghausen et al. 2015) which alone indicates a need for caution. Wider ecological problems of agrichemical farming, as opposed to organic husbandry that includes earthworms as essential farm stock, are summarized by Blakemore (2018a) and by Martinez et al. (2021).
Transitioning to agriculture methods free of synthetic fertilizer or pesticides, i.e., 100% organic, is seen as an important objective in Europe (Jacquet et al. 2022). At the same time, terms such as “regenerative” or “agroecology” are often being misappropriated by chemical industry and research interests with promotion of unproven or harmful methods such as chemical no-till (using glyphostate) or excessive marketing of unproven charcoal “char” rather than regular and much more natural compost/mulch (Beste 2022).
An EU study showed European food self-sufficiency if converted wholly organic with reduced meat (www.cnrs.fr/en/organic-farming-could-feed-europe-2050), as was shown by Billen et al. (2021: graphical abstract). This would apply to other regions with a small shift in eating habits and political will. Thus, global HANPP (~15 Gt C/yr from Krausmann et al. 2013) “wastes” may be recycled under Howard’s (1945) “Law of Return”. Simply using vermi-composts to return nutrients and mulch to fields makes healthy soil fauna and plants as natural replacements for N-P-K and pesticides (Blakemore 2018a).
It is inevitable that synthetic N needs be reduced (viz. Rockstrom et al. 2009: “contain the flow of new reactive nitrogen to 25% of its current value”), toxic agrichemical biocides eliminated (as per Carson 1962, Diamond et al. 2015, Persson et al. 2022), and meat consumption reduced (e.g. Springman et al. 2018) in order to protect what remains of the Natural world and for our future to live comfortably as an integral part of it.
3.14. SOC Loss as the “Problem” and the “Solution” with Remedy in Organic Husbandry
A global imperative is to reverse SOC loss that ultimately leads to expanding desertification impacting many regions of the globe directly or indirectly (
Figure 22).
Reduction in tree clearance for grazing, especially reduction in sheep and goats, and reduced fires would be wise management for large areas of threatened or marginal land.
As well as North Africa, or West Central Asia, three regions obviously contending to gain from organic soil restoration are: China, USA in the North and Australia in the South as these each are single political entities. If people and policy makers had proper information about healthy food and excessive meat eating then reduced forest clearance for pasture and less grazing on marginal lands are remedial steps. As the current study shows, many questions remain about historic losses of vegetation and soil, for example: Mediterranean erosion (noted by Plato and Lucretius), timing of expansion of Saharan and other deserts, and the possibility that Australian continent was once much greener. Australia is listed as third (behind China and USA) among countries with highest recent loss of soil organic carbon (Sanderman et al. 2017, 2018). However, since it was likely much more vegetated in prehistoric times, it may overall have had the greatest loss.
Of Australian relevance are Darwin’s “Beagle Diaries” where he remarked: “In the whole country I scarcely saw a place without the marks of a fire.. large tracts of country in flames, volumes of smoke sweeping across” (Keynes 1981). The 2019–2020 bushfires across Eastern Australia released ~830 Mt of CO2 to the atmosphere (DISER 2020b soe.dcceew.gov.au/views/reference/45883), or ~0.2 Gt C. For Australia’s pre-1750 era, a revised baseline estimate of maximum above-ground woody biomass potentially held 34.2 Gt of dry matter (Roxburgh et al. 2019), or ~17.1 Gt C, compared to approximately 5.6 Gt C in 2016, which roughly translates to about 11.2 Gt of dry matter (DISER 2021d - soe.dcceew.gov.au/views/reference/45894); with non-forest vegetation including cropping lands, above-ground living biomass carbon stocks estimated as 5.9 Gt C. In an independent study, which additionally included sparse woody vegetation, Liao et al. (2020) predicted the woody biomass of Australia to be around 6.6 Gt C in 2018. Thus, from 1750 to 2018, possibly as much as (17.1 minus 6.6 =) 10.5 Gt C forest carbon was lost (soe.dcceew.gov.au/land/environment/carbon). Loss from fires occurred earlier as well.
Remarkable fossil Pleistocene tree-kangaroo finds in now desertified Nullarbor (“no trees”) plain, in Thylacoleo and Mammoth Caves of WA, hint at possibility for forest restoration, if prescribed burnings were curtailed. Prideaux & Warburton (2008, 2009) said: “despite having a remarkably similar climate to today, the Nullarbor Plain was once much better vegetated, a shift that may have been wrought by increasing frequency and/or intensity of bushfires (Prideaux et al. 2007; Warburton and Prideaux in press)”; (australiangeographic.com.au/topics/wildlife/2021/03/ancient-species-of-tree-kangaroo).
How much carbon is in Australian soils? The CSIRO’s Soil Carbon Mapping Project (Rossel et al. 2014) provides national scale representation of an average amount of organic carbon in the top 30 cm of Australian soil at 29.7 t/ha and total stock for the continent at 25.0 Gt SOC (range of 19.0–31.8 Gt). The total SOC stock in agricultural regions is 12.7 Gt (range of 9.9–15.9 Gt (agriculture.vic.gov.au/__data/assets/pdf_file/0006/857607/Soil-Carbon-Snapshot-updated-May-2022.pdf). Doubled for depth then for terrain is >100 Gt SOC stock. This is remnant soil as Chan & McCoy (2010) estimated at least 50% of original SOC stock lost in intensive Australian cropping systems; essentially, SOC has been mined. Australian agricultural soil >0.3 m deep has 12.7 Gt C, doubled for depth then terrain, is 50.8 Gt SOC remaining. If the same amount is lost it may thus be open for restoration (= 24 ppm CO2)?
In WA, Australia, most soils are low in SOC, typically 0.7–4.0% with 20–160 t C/ha (agric.wa.gov.au/measuring-and-assessing-soils/what-soil-organic-carbon). Yet when digging deeper, down to bedrock at 8 to 35 m depth, Harper & Tibbett (2019; tab. 1) found mean SOC mass densities of five WA locations in a 400–600 mm/yr rainfall zone varied from 21.8–37.5 kg C/m2 or 218–375 t C/ha, and in toto were two to five times (median 3.5 x) greater than if sampled at the same site to a typical depth of 0.5 m, viz. 5.8 C/m2 or 58 t/ha SOC. Average data from typical non-desert “Soil Sink Bank” soils have ~32 t/ha, ~55 t/ha, and ~138 t/ha SOC in the top 10 cm, 50 cm, and 500 cm, respectively.
In an earlier 26-year reforesting study in WA (Harper et al. 2012), SOC stores to 0.3 m depth ranged 33–55 t/ha with no statistical differences detected between trees and adjacent crop and pasture rotation farmland at that time. Yet, reforested plots contained additional tree biomass carbon (23–60 t/ha from above-ground biomass with 1.2 x factor for roots added – note that from the data herein, root biomass is likely closer to 100% of the above-ground plant mass) and in surface litter (19–34 t/ha). When SOC is upped ~3.5 x for depth plus litter and roots added, total soil carbon is likely 280–327 t/ha, with means ~7.5 x more than tree carbon biomass alone. This ratio is slightly less than the approximate global above-ground:soil biomass ratio of 1:10 as noted in the present study (also Blakemore 2020: fig. 1 - orgprints.org/id/eprint/38139/1/Veop-4.pdf). Terrain further doubles soil/plant totals when sampled sites are extrapolated to regional areas.
A meta-analysis of the impact of land-use change on SOC concentrations by Guo & Gifford (2002) had found SOC stocks increased on average by 19% after the transition from crop to pasture, while Conant et al. (2017) provided soil carbon increase figures of ~0.87 t/ha/yr. Their fig. 1 shows summary of positive or negative SOC events (
Figure 23).
Figure 23 compares to Buringh’s (1984) data; presumably, with time, some 50% SOC loss from deforestation may be restored via reduction in fires, less red meat consumption that clears forests for crop stockfeeds, plus revival of a modern organic recycling ethos.
In a semiarid region of China, Gan et al. (2014: fig. 1) found SOC increase with 30 yrs better farming practices. Zhang et al. (2017) had higher crop footprints in China than in other countries, such as the United States, Canada and India, with improvements in SOC using different techniques in crops (e.g., maize, wheat, or rice). An important factor for carbon sequestration included return of straw to fields (+41–90%), as Howard suggested.
As another example of restoration/reclamation, in Egypt’s desert a reported 30 yrs of organic agriculture with compost and crop rotations increased SOC stocks to 0.5 m from 4 to 29–32 tonnes, a raise of 25–28 t/ha SOC or ~1 t C/ha/yr (Luske & van der Kamp 2009).
In Portugal, Terraprima pastures stored up to three times as much SOM/SOC ten years after transitioning to more natural farming (Watson 2010, Jones 2010 - amazingcarbon.com/PDF/JONES-SoilCarbon&AgricultureREVISED(18May10).pdf).
A stark possibility that Australia and other arid or Mediterranean climates can be re-vegetated needs to be accompanied by a rationale to reduce fires, lessen grazing of stock on marginal land or forest clearance for pastures or crops. As well as providing shade and windbreaks, evapo-transpiration especially from deep-rooted forests provides 60–95% of rainfall (en.wikipedia.org/wiki/File:HumanIntegratedWaterCycle_(2).jpg, vermecology.wordpress.com/2021/05/27/h2o/, Trenberth et al. 2007: fig. 1). Specifically, some 60% of land precipitation evaporates to the atmosphere; this figure exceeds 95% in arid climates (Zhan et al. 2019). Allowing natural tree regrowth is a self-perpetuating and expanding process helping to restore biotically rich topsoils and reverse desertification.
3.15. Soils Critically Undervalued and Overlooked by UN’s SDGs
UN’s SDGs (
unstats.un.org/sdgs/report/2020/progress-summary-for-SDG-targets/) failed most 2020 targets and will likely fail those set for its Agenda 2030. The present study clearly demonstrates an urgent need for context and triage in evaluating global issue priorities and goals. Especially soil is ignored at our peril. SDGs focuses on Ocean or Water and Life
on Land, but only mention “
soil” twice in passing. A complete re-evaluation and review is required for chance of truly meaningful benefits (
Figure 24).
The issue of balanced critical review in soil considerations – specifically deficits with the SDGs, and excesses with “char” or 4p1000.org hype – were raised and remedies offered by Baveye (2020a, b). Unfortunately, unchallenged is Marine misinformation hyperbole for personal or political gain that is orders of magnitude worse and diverts progress. Such distraction from soil is summarized in a quote from Koch et al. (2016: 3-4): “Discussions around biodiversity loss seldom refer to soil even though soil contains the most diverse and complex ecosystems on the planet. Soils contain over 98 per cent of the genetic diversity in terrestrial ecosystems (Fierer et al., 2007) however soil biodiversity is not addressed in the Global Biodiversity Outlook (GBO-3) from the UN Convention on Biological Diversity (Secretariat of the CBD, 2010), and is not referred to in the popular International Union for Conservation of Nature (IUCN) Red List of Threatened Species (IUCN, 2012). Recent attempts to develop a global framework for assessing planetary resources also fail to recognize the vital role of soil in the biosphere. The Stockholm Resilience Centre led an effort to define the key planetary boundaries that face anthropogenic pressure (Rockstrom et al., 2009). This important work is influential in current reviews of sustainable development, but does not address soil as a critical contributor to buffering the thresholds of those boundaries.” This misguided situation is little changed today at most recent International events (COP 27 for Climate Change) or (COP 15 Biodiversity) in 2022 that yet again mainly ignored soils and focused on Forests, FFs, Atmosphere or Oceans.
Slightly more encouraging is EU’s mission to tackle challenges of meeting SDG goals (ec.europa.eu/eip/agriculture/en/news/launch-eu-mission-soil-deal-europe) which includes soil as an issue, albeit their least important. [Current author’s comments added]:
Adaptation to Climate Change: [current report shows this depends on soil];
Cancer: [rather than genetic, most cancers have environmental causes not least from poisoned foods, soil, water & air mainly from toxic agrichemicals (vermecology.wordpress.com/2018/05/27/wormageddon-destruction-in-our-soils/)];
Restore our Ocean and Waters by 2030; [less important/urgent than soil]
100 Climate-Neutral and Smart Cities by 2030; [with 100% organic food?]
A Soil Deal for Europe: 100 living labs and lighthouses to lead transition towards healthy soils by 2030. [A commendable goal that may also be met with several 100% organic agro-ecological Soil Ecology Institutes working on a Context & Triage basis, as proposed herein. Something less maritime than “lighthouses” may be such as “Boden-beacons”. Some of their Soil initiatives are - ec.europa.eu/eip/agriculture/en/news/safeguarding-our-soils].
4. CONCLUSIONS
Earth’s major and interlinked problems – Erosion, Extinctions, Climate, Pollution – are mainly caused by poor agricultural practices that may be reversed or remedied with restoration of proper farming following simple principles such as conserving earthworms and composting. Key outcomes of the current study are summarized in
Table 5.
* Reiner’s (1973) estimate fifty years ago of ~9,120 Gt SOC is proving almost correct. ** Values in Tabs. 1–4, Figs. 5–6 and text. Fire values of Figs. 5–6, as supported by text, add 4–8 Gt C/yr to the mean ~12 Gt C LUC/SOC loss to give 16–20 Gt C/yr. See also veop.files.wordpress.com/2020/06/veop-4-5.pdf. Note: LUC loss of 410 Gt C/yr in 172 yrs is ~2.3 Gt C/yr, but if agricultural intensification increase in last 65 years caused half loss (205/65 =) 3.2 Gt C/yr (cf. Tab. 3, Fig. 6). SOC loss median in Tab. 4 of 9 Gt SOC/yr (cf. Fig. 6) in median 400 Gt soil erosion gives ~2.25% similar to 2.5% mean in METHODS. Q.E.D. *** Agrichemical earthworms are depleted by ~80%, so a prospect is for x 5 increase.
The persistent idea that cutting FF will equally cut CO2 was disproven by COVID-19 Shutdown that equated to 5–20% less FF in 2020–2021 but had little or no effect on CO2 and other GHGs that increase due to bad agriculture and further (deliberate or accidental) forest burning. As Forster et al. (2020: fig. 4) summarized: despite Shutdown restrictions cutting fossil fuel CO2 emissions, atmospheric carbon and global temperatures continued to rise. Cooling and warming effects of various NOx and CO2 emissions more-or-less cancelled out in their simulations, although on balance they expected a small warming effect. This conclusion was also arrived at by NOAA (web.archive.org/web/20201003215958/https:/www.esrl.noaa.gov/gmd/ccgg/covid2.html; see also - vermecology.wordpress.com/2020/08/31/barrow/).
Evidence mounts that best means to lower greenhouse gasses (GHGs) is via SOC sequestration combined with synthetic N fertilizer reduction to safely drawdown CO2 to desired levels, with no penalties, under a safe ozone layer ‘parasol’. An acceptable aerosol pollution level must seemingly be maintained because rapid reduction in air pollution is expected to result in instant increase in irradiation with a lower aerosol radiative forcing of smog plus its cloud seeding effects raising temperatures by several °C degrees (Peace et al. 2020: fig. 4) that may further affect farming in unpredictable ways.
Robust organic farming in essence is humic SOC farming aiming for healthy, diverse, earthworm-rich field soils that alone fixes many problems. A simple solution to SOC loss may be found in soil humus restoration via vermi-composting. A most urgent need is for completion of a global earthworm eco-taxonomic inventory with assessment of conservation status in order to monitor – and to mitigate – changes in soil health or carbonization status as priority tasks. Yet there is little support for organic research and no Soil Ecology Institute to conduct such work. If founded, Soil Ecology Institute tasks may be re-evaluation of data in Rodin et al. (1975: tabs. 1–6) providing thorough and detailed assessment of global phytomass and NPP – both above- and below-ground – that, after 50 years, now converge with current estimates determined herein with terrain (
Table 6).
* Current paper has Phytomass + litter of ~2,620 Gt C, compared to a total global Living Matter in Rodin et al. (tab. 5) of 1,211.5 Gt C that, when doubled is 2,423 Gt C and is close to current calculations. Q.E.D. ** Current NPP is ~220 Gt C/yr (with ~25% boreal?) while Rodin et al. allocate 80 percent NPP in tropics and subtropics, just 10 percent in boreal/polar. Ambiguity in their NPP is whether “total standing crop” includes all “underground parts” in roots, or not. Also, as Li et al. (2017) had 21.5% NPP increase in 50 years, extrapolating this rate may similarly up their 1970 value of >86 Gt to now total >104 Gt C/yr that, if doubled for terrain to >208 Gt C/yr is within bounds of current NPP calculations. Q.E.D. *** In their text, Ocean NPP is 47 to 72 Gt dry mass/yr = 23.5–36 Gt C/yr (mean ~30), Lakes & Rivers is 0.5 Gt C/yr. Mangrove NPP in their tab. 3 is just 0.25 Gt C/yr a trivial contribution at 0.1% of NPP. As noted in an Ocean section above, realistic estimates put Ocean NPP range 10–30 Gt C/yr (mean ~20) to give overall global total of ~240 Gt C/yr which is a slight reduction from a previous estimate (Blakemore 2018b) of 270 Gt C/yr.
Self-replicating feedbacks of SOC loss from forest clearance and Permafrost melt with inevitable increases in atmospheric carbon and climate change will shift habitable Landscapes. Yet with proper ecological/Permaculture measures, topsoil may be restored in a reasonably short-time. Extinctions however are irreversible and eternal. Thus the most urgent imperative is to immediately halt poisoning of soils to preserve and protect what remains of their biota as a priority. Albeit plant standing stock, SOC, and global NPP rates are raised due to terrain, it is increasingly obvious, and undeniable, that the terrestrial ecosystem is being stretched and is collapsing – especially our soils. A receding opportunity is to focus all resources on saving soils by restoring organic husbandry and eliminating toxic chemicals. Rather than chemical convenience, realization is for a complex, aesthetic and vibrant living Earth with more natural options in all our endeavours. As well as meat reduction, a first step to restoration – open to all, anywhere, at any scale – is recycling via non-toxic compost. As Sir Albert Howard showed, along with humic carbon stabilization (minus CO2 respiration), compost nitrogen increases above added constituents due to N-fixing microbes, thus providing a basis for sustainable Farm soils.
Data presented in the current study are best estimates but all are open for review. The duty of a Scientist is to correct their own and others’ errors in light of new facts. Regarding topographic soil surface area, improvements and refinement of terrain are welcomed but, thus far, no study has improved upon topographical area nor invalidated a non-flat Earth theory. Any detractors are invited to provide their own detailed estimate of topography at finest scales, or else to prove – or to at least to reflect upon – support for a “mirror-flat” Earth. Theory may be tested by proper in-depth surveys of small islands.
Use of fossil fuels will likely prevail, but better alternatives for limitless, non-polluting, free-energy are in Geothermal and (where rain falls or rivers flow) Trompe power as both provide pneumatic energy for cars, industry, etc. that, combined with Organic Farming, show us an honest, true trail. Commentary on such issues are postponed for appropriately directed and further context-triaged research in due course.
Figure 1.
Flat land areas (after Blakemore 2018b: fig. 4), now more than doubled to >30–64 Gha. Note that >99% of human food comes from land and that aquaculture often uses crop feedstocks too. Urban areas (with infractructure such as roads or railways) occupy only around 1% of total land surface with total “Built-up area” defined as cities, towns, villages and human infrastructure.
Figure 1.
Flat land areas (after Blakemore 2018b: fig. 4), now more than doubled to >30–64 Gha. Note that >99% of human food comes from land and that aquaculture often uses crop feedstocks too. Urban areas (with infractructure such as roads or railways) occupy only around 1% of total land surface with total “Built-up area” defined as cities, towns, villages and human infrastructure.
Figure 2.
After Lal (2006: fig. 3.2), global SOC loss reallocations from agriculture and natural processes (Pg = Gt). Terrain doubled values are in red. Subtracting Redistribution carbon gives CO2 Emission proportions >66% and, if Redistribution SOC in turn erodes, this % increases recursively.
Figure 2.
After Lal (2006: fig. 3.2), global SOC loss reallocations from agriculture and natural processes (Pg = Gt). Terrain doubled values are in red. Subtracting Redistribution carbon gives CO2 Emission proportions >66% and, if Redistribution SOC in turn erodes, this % increases recursively.
Figure 3.
Carbon Cycle Concept (IPCC 2000: fig.1.2 - archive.ipcc.ch/ipccreports/sres/land_use). Disturbance is “both from natural and anthropogenic sources (e.g., harvest)”. Net Ecosystem Production (NEP) adds to SOC organic matter often as Net Biome Production (NBP). Allowance for terrain more than doubles all these values, with NPP now at ~220 Gt C/yr (Blakemore 2018b, 2020c).
Figure 3.
Carbon Cycle Concept (IPCC 2000: fig.1.2 - archive.ipcc.ch/ipccreports/sres/land_use). Disturbance is “both from natural and anthropogenic sources (e.g., harvest)”. Net Ecosystem Production (NEP) adds to SOC organic matter often as Net Biome Production (NBP). Allowance for terrain more than doubles all these values, with NPP now at ~220 Gt C/yr (Blakemore 2018b, 2020c).
Figure 4.
Microbiomes from Locey & Lennon (2016: fig. 3) and Zhao et al. (2022: fig. 3A) that consider some internal enterobiota, parasites or symbionts living within or upon hosts. Wastewater, air, freshwater, sea and soil biota are included. Soil microbe data is revised upwards in
Table 2.
Figure 4.
Microbiomes from Locey & Lennon (2016: fig. 3) and Zhao et al. (2022: fig. 3A) that consider some internal enterobiota, parasites or symbionts living within or upon hosts. Wastewater, air, freshwater, sea and soil biota are included. Soil microbe data is revised upwards in
Table 2.
Figure 5.
Organic carbon cycle from IPCC (2001: fig. 3–1 WGI-TAR1) updated by Blakemore (2018b) in red (“τ” is estimated turnover time in years). Plant biomass turnover has been halved by LUC in the current era from 13.7 to 7.1 years (Erb et al. 2016). Lesser “inert” Carbon may include calcium carbonates of biotic origin (e.g., earthworm calciferous glands or mollusc shells) and a small amount of charcoal. Rather than 150 Gt, “inert” soil inorganic carbon (SIC) totals 695–1,738 Gt C in Naorem et al. (2022: fig. 1); doubled for terrain, values to 1 m now increase to 1,390–3,476 Gt SIC; to 2m depth SIC total is >2,300 Gt (Zamantian et al. 2021) which, when doubled, is >4,600 Gt C.
Figure 5.
Organic carbon cycle from IPCC (2001: fig. 3–1 WGI-TAR1) updated by Blakemore (2018b) in red (“τ” is estimated turnover time in years). Plant biomass turnover has been halved by LUC in the current era from 13.7 to 7.1 years (Erb et al. 2016). Lesser “inert” Carbon may include calcium carbonates of biotic origin (e.g., earthworm calciferous glands or mollusc shells) and a small amount of charcoal. Rather than 150 Gt, “inert” soil inorganic carbon (SIC) totals 695–1,738 Gt C in Naorem et al. (2022: fig. 1); doubled for terrain, values to 1 m now increase to 1,390–3,476 Gt SIC; to 2m depth SIC total is >2,300 Gt (Zamantian et al. 2021) which, when doubled, is >4,600 Gt C.
Figure 6.
Organic C cycle relevant to climate, modified from Houghton (
2007: fig. 1) updated from Blakemore (2020a, b, c -
https://vermecology.wordpress.com/2020/01/16/solum-solace/) as detailed herein. Volatile Organic Compounds (VOC), mainly released by plants, are not included but may add a further 1–2 Gt C/yr to atmospheric total (Guenther et al. 1995). Omitted too is relatively minor methane C emitted mainly by cattle and other agriculture (IPCC 2001 -
www.ipcc.ch/report/ar5/wg1/carbon-and-other-biogeochemical-cycles/methane_test-3/feed/). Mostly natural soil respiration/decomposition (SR) of 200 Gt C/yr is >20 x FF emissions of 9.4 Gt C/yr, matched by 9.2 Gt C/yr SOC loss (cf. median of ~9 Gt SOC/yr in
Table 4) largely from (bad) farming methods, plus 3.2 from Vegetation = ~12.4 Gt C/yr. Adding Fire (4–8 Gt C) to LUC (~12.4 Gt C) gives total Land emissions range ~16–20 Gt C Gt C/yr that is also about double the FF emissions. [Net ocean fluxes are just ∼±3 Gt C/yr (Koren et al. 2019: fig. 1) or ∼±1–4% (Piao et al. 2019: fig. 9). Note too how Surface ocean DOC is just 700 Gt C and sea DIC is only 25 Gt C compared to Soil].
Figure 6.
Organic C cycle relevant to climate, modified from Houghton (
2007: fig. 1) updated from Blakemore (2020a, b, c -
https://vermecology.wordpress.com/2020/01/16/solum-solace/) as detailed herein. Volatile Organic Compounds (VOC), mainly released by plants, are not included but may add a further 1–2 Gt C/yr to atmospheric total (Guenther et al. 1995). Omitted too is relatively minor methane C emitted mainly by cattle and other agriculture (IPCC 2001 -
www.ipcc.ch/report/ar5/wg1/carbon-and-other-biogeochemical-cycles/methane_test-3/feed/). Mostly natural soil respiration/decomposition (SR) of 200 Gt C/yr is >20 x FF emissions of 9.4 Gt C/yr, matched by 9.2 Gt C/yr SOC loss (cf. median of ~9 Gt SOC/yr in
Table 4) largely from (bad) farming methods, plus 3.2 from Vegetation = ~12.4 Gt C/yr. Adding Fire (4–8 Gt C) to LUC (~12.4 Gt C) gives total Land emissions range ~16–20 Gt C Gt C/yr that is also about double the FF emissions. [Net ocean fluxes are just ∼±3 Gt C/yr (Koren et al. 2019: fig. 1) or ∼±1–4% (Piao et al. 2019: fig. 9). Note too how Surface ocean DOC is just 700 Gt C and sea DIC is only 25 Gt C compared to Soil].

Figure 7.
Like an iceberg, the great bulk of active carbon is concealed below Land’s surface. Vegetation, soil carbon and microbes allocations adapted from Crowther et al. (2019: fig. 2). Authors were explicit: “aboveground plant biomass (green) and soil carbon stocks (brown)” of ~560 and ~1,500 Gt, respectively. Both are now corrected in a revised figure above with further explanation in text.
Figure 7.
Like an iceberg, the great bulk of active carbon is concealed below Land’s surface. Vegetation, soil carbon and microbes allocations adapted from Crowther et al. (2019: fig. 2). Authors were explicit: “aboveground plant biomass (green) and soil carbon stocks (brown)” of ~560 and ~1,500 Gt, respectively. Both are now corrected in a revised figure above with further explanation in text.
Figure 9.
Global biodiversity concepts advanced rapidly following PCR and genetic sequencing. Sources: Blakemore (2018b: fig. 15); https://vermecology.wordpress.com/2021/06/20/tol/ ; https://vermecology.wordpress.com/2022/08/04/different-f3/.
Figure 9.
Global biodiversity concepts advanced rapidly following PCR and genetic sequencing. Sources: Blakemore (2018b: fig. 15); https://vermecology.wordpress.com/2021/06/20/tol/ ; https://vermecology.wordpress.com/2022/08/04/different-f3/.
Figure 12.
Multiple soilscape benefits of earthworm activities demonstrated in a simple experiment. By virtue of manifest skills, the humble earthworm is greatest threat to the agrichemical industry.
Figure 12.
Multiple soilscape benefits of earthworm activities demonstrated in a simple experiment. By virtue of manifest skills, the humble earthworm is greatest threat to the agrichemical industry.
Figure 13.
Revised after Tuma et al. (2009: fig. 1). Values are mostly doubled by terrain for all groups from (planimetrically-flat surface) biome area surveys, except Livestock (0.1 Gt C) and Humans (0.06 Gt C) based on audited counts. Reassessment has ants as 0.024 Gt C, termites 0.15 Gt C; while neglected earthworms are ~2.3–3.6 Gt greatly exceeding all others’ ~0.5 Gt C. Ironically, as terrain doubles it (Blakemore 2018b), extinctions may halve soil faunal biomass (Blakemore 2018a).
Figure 13.
Revised after Tuma et al. (2009: fig. 1). Values are mostly doubled by terrain for all groups from (planimetrically-flat surface) biome area surveys, except Livestock (0.1 Gt C) and Humans (0.06 Gt C) based on audited counts. Reassessment has ants as 0.024 Gt C, termites 0.15 Gt C; while neglected earthworms are ~2.3–3.6 Gt greatly exceeding all others’ ~0.5 Gt C. Ironically, as terrain doubles it (Blakemore 2018b), extinctions may halve soil faunal biomass (Blakemore 2018a).
Figure 14.
Source OWiD. Associated with a possible increase in plant growth following extinction of quaternary megafaunal herbivory may be an offset of increased vegetation fires by humans.
Figure 14.
Source OWiD. Associated with a possible increase in plant growth following extinction of quaternary megafaunal herbivory may be an offset of increased vegetation fires by humans.
Figure 15.
After Koren et al. (2019: fig. 4).FAL = Flux air<->leaf in North and South with C3/C4.
Figure 15.
After Koren et al. (2019: fig. 4).FAL = Flux air<->leaf in North and South with C3/C4.
Figure 16.
Source: vermecology.files.wordpress.com/2020/10/image-4.png. Summary of NPP and Global Carbon Cycle from CO2 emissions data based upon Barrow CO2 “bounce”. Note: Expensive BECCS/CCS sequestration schemes have minutely insignificant drawdown relative to SOC flux.
Figure 16.
Source: vermecology.files.wordpress.com/2020/10/image-4.png. Summary of NPP and Global Carbon Cycle from CO2 emissions data based upon Barrow CO2 “bounce”. Note: Expensive BECCS/CCS sequestration schemes have minutely insignificant drawdown relative to SOC flux.
Figure 17.
Oxygen vs. Carbon fluxes. Source: vermecology.wordpress.com/2021/05/27/h2o/. Cf. Keeling & Manning (2014: fig. 2); also see wernerantweiler.ca/blog.php?item=2015-06-01 that corrects O2 per meg/yr decline to 10 x CO2 ppm/yr increase (i.e., ratio O2 :CO2 of 10:1). CDIAC (https://cdiac.ess-dive.lbl.gov/trends/oxygen/) has O2 decline of 19 per meg/yr or -4 ppm/yr O2.
Figure 17.
Oxygen vs. Carbon fluxes. Source: vermecology.wordpress.com/2021/05/27/h2o/. Cf. Keeling & Manning (2014: fig. 2); also see wernerantweiler.ca/blog.php?item=2015-06-01 that corrects O2 per meg/yr decline to 10 x CO2 ppm/yr increase (i.e., ratio O2 :CO2 of 10:1). CDIAC (https://cdiac.ess-dive.lbl.gov/trends/oxygen/) has O2 decline of 19 per meg/yr or -4 ppm/yr O2.
Figure 18.
From Huang et al. (2018: fig. 4); note Land’s 16.01 Gt/yr O2 production is nearly ten times Ocean’s 1.74 Gt/yr outgassing, or >90%. When doubled for terrain this is nearly 20 x or >95%! Cf. vermecology.wordpress.com/2021/01/05/pp-bs/; vermecology.wordpress.com/2021/05/18/o2/.
Figure 18.
From Huang et al. (2018: fig. 4); note Land’s 16.01 Gt/yr O2 production is nearly ten times Ocean’s 1.74 Gt/yr outgassing, or >90%. When doubled for terrain this is nearly 20 x or >95%! Cf. vermecology.wordpress.com/2021/01/05/pp-bs/; vermecology.wordpress.com/2021/05/18/o2/.
Figure 19.
Wuepper et al.’s (2021: summary fig.) of anthropogenic carbon “
debt”. Stated “
above-ground” value of 871 -> 601 Gt C (that may be doubled for terrain) excludes roots and is above usual plant biomass values around 550 Gt C (now 1,100 Gt C with terrain); thus their 270 Gt C debt may also be doubled to 540 Gt C. All three of their tree graphics omit roots (ditto their paper and supplementary fail to consider “
roots”). SDG 15 LIFE ON LAND (sic) almost entirely ignores LIFE
IN SOIL as its major failure. Figure 8 may be compared to data in
Tables 1, 3 and
Figure 7.
Figure 19.
Wuepper et al.’s (2021: summary fig.) of anthropogenic carbon “
debt”. Stated “
above-ground” value of 871 -> 601 Gt C (that may be doubled for terrain) excludes roots and is above usual plant biomass values around 550 Gt C (now 1,100 Gt C with terrain); thus their 270 Gt C debt may also be doubled to 540 Gt C. All three of their tree graphics omit roots (ditto their paper and supplementary fail to consider “
roots”). SDG 15 LIFE ON LAND (sic) almost entirely ignores LIFE
IN SOIL as its major failure. Figure 8 may be compared to data in
Tables 1, 3 and
Figure 7.
Figure 20.
Sources IPCC (2022: fig. SPM.1D) exactly the same as IPCC (2019a: fig. SPM.1D); also from vermecology.wordpress.com/2020/01/16/solum-solace/. Note triple yields correlate more with doubled irrigation (water also used more efficiently) than with 9 x increase in N fertilizer.
Figure 20.
Sources IPCC (2022: fig. SPM.1D) exactly the same as IPCC (2019a: fig. SPM.1D); also from vermecology.wordpress.com/2020/01/16/solum-solace/. Note triple yields correlate more with doubled irrigation (water also used more efficiently) than with 9 x increase in N fertilizer.
Figure 21.
Proportionate farm variables extracted mainly from FAO data; source - vermecology.wordpress.com/2020/10/16/nitrogen-necrosis/. Haber-Bosch (H-B) Ammonium fertilizers expanded with WW1 surplus munitions (green). Novel WW2 bio-weapons use, despite Dr Rachel Carson’s warnings and EPA formation, also grew exponentially (red). Yields (black) and people (yellow) correlate more with efficient irrigation (blue) than N fertilizer or biocide overuse.
Figure 21.
Proportionate farm variables extracted mainly from FAO data; source - vermecology.wordpress.com/2020/10/16/nitrogen-necrosis/. Haber-Bosch (H-B) Ammonium fertilizers expanded with WW1 surplus munitions (green). Novel WW2 bio-weapons use, despite Dr Rachel Carson’s warnings and EPA formation, also grew exponentially (red). Yields (black) and people (yellow) correlate more with efficient irrigation (blue) than N fertilizer or biocide overuse.
Figure 22.
Red desert risk from https://en.wikipedia.org/wiki/File:Desertification_map.png with rate of expansion up to several Km per year (Balfour 1943). Notable in the map above is Brecklands a tiny vulnerable part in East Anglia UK, while Australia’s desertification is nearly entire. Natural plant succession into cooler blue areas as they warm may offset desertification to some degree.
Figure 22.
Red desert risk from https://en.wikipedia.org/wiki/File:Desertification_map.png with rate of expansion up to several Km per year (Balfour 1943). Notable in the map above is Brecklands a tiny vulnerable part in East Anglia UK, while Australia’s desertification is nearly entire. Natural plant succession into cooler blue areas as they warm may offset desertification to some degree.
Figure 23.
After Guo & Gifford (2002: fig. 1). SOC responses to various LUC events (± 95% CIs). Their meta-analysis covered 16 countries but mainly: Australia, Brazil, New Zealand, and USA.
Figure 23.
After Guo & Gifford (2002: fig. 1). SOC responses to various LUC events (± 95% CIs). Their meta-analysis covered 16 countries but mainly: Australia, Brazil, New Zealand, and USA.
Figure 24.
Source: Ame (雨) Power, vermecology.wordpress.com/2020/07/30/ame-power/. UN’s SDGs require revision to recognize the Soil foundation upon which the Biosphere depends. Society and Economy are both entirely reliant on a stable and secure Soil Ecology to support the Biosphere. Several mistakes and falsehoods in SDG14: “LIFE BELOW WATER” are exposed here - vermecology.wordpress.com/2019/07/02/the-ocean-lies-still/.
Figure 24.
Source: Ame (雨) Power, vermecology.wordpress.com/2020/07/30/ame-power/. UN’s SDGs require revision to recognize the Soil foundation upon which the Biosphere depends. Society and Economy are both entirely reliant on a stable and secure Soil Ecology to support the Biosphere. Several mistakes and falsehoods in SDG14: “LIFE BELOW WATER” are exposed here - vermecology.wordpress.com/2019/07/02/the-ocean-lies-still/.
Table 1.
Earth’s Global Carbon Budget (in Gt C) after UNEP’s (2002: tab. 2.1).
Table 1.
Earth’s Global Carbon Budget (in Gt C) after UNEP’s (2002: tab. 2.1).
| CARBON STORE |
Inactive & Active C (%) |
Active Surface C (%) |
| Sedimentary Rock (carbonate) |
65,000,000 (80.20%) |
|
| Sedimentary Rock (organic) |
16,000,000 (19.74%) |
|
| Deep Dissolved Inorganic (sea DIC) |
38,000 (0.05%) |
|
| Active Carbon at Surface |
Median ~22,000 (0.03%) |
|
| Soil Organic Carbon (SOC) * |
|
>10,000–15,000 (57%) |
| Soil Dissolved Inorganic C (DIC) * |
|
3,000–6,000 (20%) |
| Soil Biomass (biota, litter, roots) ** |
|
~2,000 (9%) |
| Above-ground Biomass on Land ** |
|
1,100 (5%) |
| Atmospheric Carbon (CO2) |
|
875 (4%) |
| Old Organic C (fossil fuels, FF) |
|
805 (3%) |
| Dissolved Organic C (sea DOC) ** |
|
0.2–700 (<2%) |
| Phytomass or Biomass in Sea ** |
|
0.085 or ~4.5 (<0.02%) |
| Biomass in Freshwater *** |
|
~1.0 (0.005%) |
| Biomass in Air **** |
|
<0.5? (0.002%) |
| TOTAL (%) |
81,060,000 (100%) |
18,000–26,000 (100%) |
Table 2.
Number, Biodiversity and Biomass of Prokaryotes in Earth’s Six Ecological Realms-of-Life.
Table 2.
Number, Biodiversity and Biomass of Prokaryotes in Earth’s Six Ecological Realms-of-Life.
| Ecological Realm |
Cells/CFUs x 1028 (%) |
Species or OTUs (%) |
Biomass Gt C (%) |
| 1. Soil * |
210 (56%) |
2.1 x 1024 (99.99%) |
~200 (55%) |
| 2. Land Superficial ** |
100 (27%) |
1012 (<0.001%) |
~100? (28%?) |
| 3a. Land Subsurface *** |
~20–60 (11%) |
<105 |
~23–31 (7%) |
| 3b. Marine Subsurface *** |
~2.9–35 (4%) |
<106 |
35 (<1.0%) |
| 4. Ocean ** |
12 (3%) |
1010 (<0.0001%) |
0.6–2.2 (0.3%) |
| 5. Aquatic on Land ** |
<0.02 ( <0.005%) |
<1010 (<0.0001%) |
0.3? (<0.1%?) |
| 6. Atmosphere **** |
(1024) |
108 – 1010 |
(0.0001?) |
| TOTAL |
~378 x 1028 (100%) |
~2 x 1024 (100%) |
~363 (100%)? |
Table 3.
Latest Reviews of Human CO2 Carbon Sources and Atmospheric Sink (in Gt C).
Table 3.
Latest Reviews of Human CO2 Carbon Sources and Atmospheric Sink (in Gt C).
| Carbon Source/Sink |
OWiD (%) |
ESSD (2022) (%) |
Current study (%) |
| LUC <1850 * |
- |
- |
(~325–357 x 2 = ~682) |
| LUC >1850 ** |
201 (31%) |
205 (31%) |
(205 x 2 =) 410 (47%) *** |
| LUC >1950 ** |
(96.5) |
- |
(96.5 x 2 = 193) |
| FF Emissions >1850 |
460 (69%) |
465 (69%) |
465 (53%) |
| Total Emissions (TE) >1850 |
661 (100%) |
670 (100%) |
875 (100%) |
| Total CO2 >1850 (% TE) |
276 Gt C(42%) |
274 Gt C (41%) |
~275 Gt C (31%) |
Table 4.
Summary of Erosion (Gt dry soil /yr) and SOC Loss (Gt C/yr), Doubled for Terrain.
Table 4.
Summary of Erosion (Gt dry soil /yr) and SOC Loss (Gt C/yr), Doubled for Terrain.
| Author |
Erosion Gt/yr |
Terrain x 2 |
SOC Gt C/yr |
Terrain x 2 (SOC%) |
| Buringh (1984: tab. 3.8) * |
- |
- |
2.5–7.4 * |
5.0–14.8 |
| Lal (1995: fig. 2) |
190 |
380 |
5.7 |
11.4 (3.0%) |
| Lal (2006: fig. 3.2) |
- |
- |
4.0–6.1 |
8.0–12.2 |
| Lal (2006: tab. 3.2) |
200 |
400 |
>1.4 |
>2.8 (0.7%) |
| Pimentel & Burgess (2013) |
75 |
150 |
- |
- |
| FAO (2015a, b) (water+wind) |
~<203 |
~<406 |
- |
- |
| Gao et al (2017) (rivers only) |
- |
- |
>4.0–6.0 |
>8.0–12.0 |
| Alves et al. (2018: fig. 3) |
- |
- |
2.0–6.0 |
4.0–12.0 |
| Lal (2020, 2022) (water only) |
36.6 |
73.2 |
1.3 |
2.6 (3.6%) |
| RANGES (Approximate) |
37–203 |
73–406 |
>1.4–7.4 |
>2.8–14.8 (2.4%) ** |
Table 5.
Summary of Approximated Results.
Table 5.
Summary of Approximated Results.
| Factor |
Prior Range |
Median |
With Terrain (x 2) |
Source |
| Total SOC + Peat Gt C |
1,417–9,120 * |
5,268.5 |
>10,000–15,000 * |
Blakemore 2020c & Current study |
| “Official” 2 m SOC Gt C |
2,815–5,796.1 |
4,305.5 |
5,630–11,600 |
Wang et al. 2022 |
| Peat Gt C |
500–1,123 |
~800 |
1,123 (no terrain) |
Loisel et al. 2021 |
| Aboveground Tree Gt C |
450–650 |
550 |
1,100 |
Blakemore 2018b |
| Roots Gt C |
300–600 |
450 |
916 |
Current |
| Litter Gt C |
100–400 |
250 |
600 |
Current |
| Soil Microbes Gt C |
23–50 |
40 |
200 |
Blakemore 2022 |
| Soil Fungi Gt C |
12–15 |
13.5 |
30 |
Current |
| Land NPP (≈ SR) Gt C/yr |
55–300 |
110 |
~220 |
Blakemore 2018b, 2019a & Current |
| Ocean NPP Gt C/yr |
10–30 |
20 |
20 |
Current |
| LUC/SOC + Fire Gt C/yr |
~2–4 + 2–4 |
~6 |
12+4–8 = 16–20 ** |
Current ** |
| Soil Erosion Gt dry/yr |
35–200 |
~100 |
200–600 ** |
Current ** |
| Fossil Fuel (FF) Gt C/yr |
9–10 |
9.5 |
9.5 |
IPCC, ESSD 2022 |
| Earthworms Gt C |
1–2 |
1.5 |
2–4 *** |
Blakemore 2018a |
Table 6.
Phytomass and NPP from Rodin et al. (1975: tabs. 1–5 - https://nap.nationalacademies.org/read/20114/chapter/3) with Values Doubled for Terrain.
Table 6.
Phytomass and NPP from Rodin et al. (1975: tabs. 1–5 - https://nap.nationalacademies.org/read/20114/chapter/3) with Values Doubled for Terrain.
| Domain |
Phytomass Gt C |
Terrain x 2 Mass |
NPP Gt C/yr |
Terrain x 2 NPP |
| All Continents |
1,200 (>99.9%) |
2,400 (>99.9%) * |
>86 (>74%) |
>172 (>90%) ** |
| Ocean |
0.085 (0.0%) |
0.085 (0.0%) |
~30 (<26%) |
~20 (<10%) *** |
| Lakes & Rivers |
0.02 (0.0%) |
0.02 (0.0%) |
0.5 (0.4%) |
0.5 (0.26%) *** |
| TOTAL |
1,200 (100%) |
2,400 (100%) |
116.5 (100%) |
192.5 (100%) |