Submitted:
22 February 2023
Posted:
23 February 2023
You are already at the latest version
Abstract
Keywords:
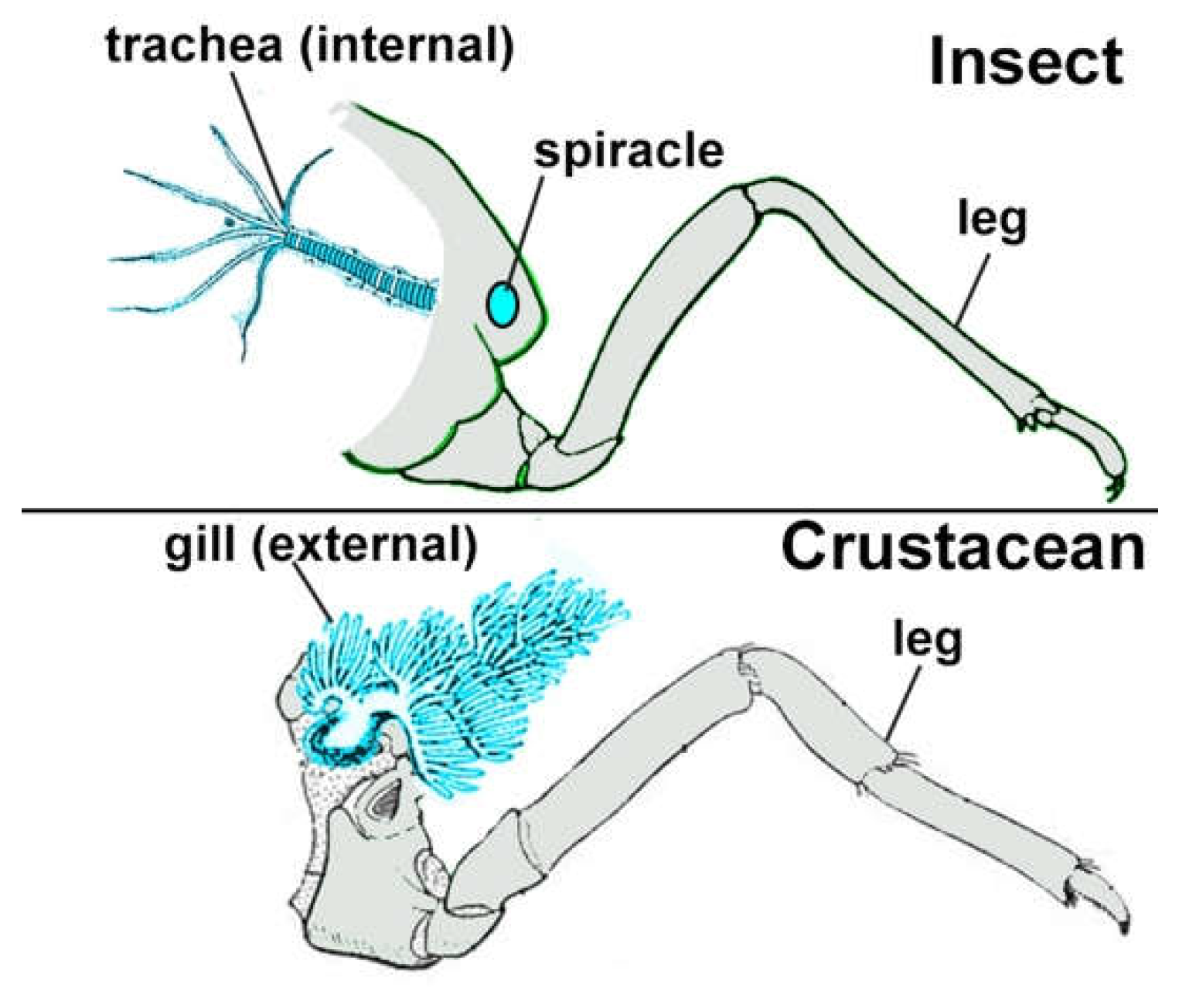
1. Introduction
2. Results
3. Discussion
Author Contributions
Acknowledgements
Declaration of interests
References
- Matsuda, R. Morphology and evolution of the insect abdomen: with special reference to developmental patterns and their bearings upon systematics. (Pergamon Press, 1976).
- Kobayashi, Y.; Niikura, K.; Oosawa, Y.; Takami, Y. Embryonic development of Carabus insulicola (Insecta, Coleoptera, Carabidae) with special reference to external morphology and tangible evidence for the subcoxal theory. J. Morphol. 2013, 274, 1323–1352. [Google Scholar] [CrossRef] [PubMed]
- Brusca, R.C.; Brusca, G.J. Invertebrates. (Sinauer Associates Incorporated, 2003).
- Anderson, D.T. Embryology And Phylogeny In Annelids And Arthropods. (Pergamon Press Ltd., 1973).
- Roonwal, M.L. Studies on the embryology of the African migratory locust, Locusta migratoria migratorioides Reiche and Frm.(Orthoptera, Acrididae). II. Organogeny. Philos. Trans. R. Soc. London. Ser. B: Biol. Sci. 1937, 227. [Google Scholar]
- Ikeda, Y.; Machida, R. Embryogenesis of the dipluran Lepidocampa weberi Oudemans (Hexapoda, Diplura, Campodeidae): external morphology. J. Morphol. 1998, 237, 101–115. [Google Scholar] [CrossRef]
- Machida, R. External features of embryonic development of a jumping bristletail, Pedetontus unimaculatus Machida (Insecta, Thysanura, Machilidae). J. Morphol. 1981, 168, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Uchifune, T.; Machida, R. Embryonic development of Galloisiana yuasai Asahina, with special reference to external morphology (insecta: Grylloblattodea). J. Morphol. 2005, 266, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Deuve, T. What is the epipleurite? A contribution to the subcoxal theory as applied to the insect abdomen. Ann. Soc. Entomol. Fr. 2018, 54, 1–26. [Google Scholar] [CrossRef]
- Bruce, H.S.; Patel, N.H. Knockout of crustacean leg patterning genes suggests that insect wings and body walls evolved from ancient leg segments. Nat. Ecol. Evol. 2020, 4, 1703–1712. [Google Scholar] [CrossRef]
- Bruce, H.S. How to align arthropod leg segments. bioRxiv. 2021. [CrossRef]
- Bruce, H.S.; Patel, N.H. The Daphnia carapace and other novel structures evolved via the cryptic persistence of serial homologs. Curr. Biol. 2022, 32. [Google Scholar] [CrossRef]
- Suzuki, Y.; Palopoli, M. Evolution of insect abdominal appendages: are prolegs homologous or convergent traits? Dev. Genes Evol. 2001, 211, 486–492. [Google Scholar] [CrossRef]
- Panganiban, G.; Nagy, L.; Carroll, S.B. The role of the Distal-less gene in the development and evolution of insect limbs. Curr. Biol. 1994, 4, 671–675. [Google Scholar] [CrossRef]
- Almudi, I.; et al. Genomic adaptations to aquatic and aerial life in mayflies and the origin of insect wings. Nat. Commun. 2020, 11, 2631. [Google Scholar] [CrossRef] [PubMed]
- Bank, S.; et al. A tree of leaves: Phylogeny and historical biogeography of the leaf insects (Phasmatodea: Phylliidae). Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Motyka, M.; et al. Conspicuousness, phylogenetic structure, and origins of Müllerian mimicry in 4000 lycid beetles from all zoogeographic regions. Sci. Rep. 2021, 11, 5961. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.L.; DeCamillis, M.; Bennett, R.L. Distinct roles of the homeotic genes Ubx and abd-A in beetle embryonic abdominal appendage development. Proc. Natl. Acad. Sci. USA 2000, 97, 4504–4509. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.M.; Grbić; M. Cell Lineages in Larval Development and Evolution of Holometabolous Insects. in The Origin and Evolution of Larval Forms 275–300 (Elsevier, 1999). [CrossRef]
- Galant, R.; Carroll, S.B. Evolution of a transcriptional repression domain in an insect Hox protein. Nature 2002, 415, 910–913. [Google Scholar] [CrossRef]
- Jockusch, E.L.; Smith, F.W. Hexapoda: Comparative Aspects of Later Embryogenesis and Metamorphosis. in (null) 111–208 (Springer Vienna, 2015). [CrossRef]
- Estella, C. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr. Top. Dev. Biol. 2012, 98, 173–198. [Google Scholar]
- Bowsher, J.H.; Nijhout, H.F. Partial co-option of the appendage patterning pathway in the development of abdominal appendages in the sepsid fly Themira biloba. Dev. Genes Evol. 2010, 219, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernandez, J.; et al. Pancrustacean Evolution Illuminated by Taxon-Rich Genomic-Scale Data Sets with an Expanded Remipede Sampling. Genome Biol. Evol. 2019, 11, 2055–2070. [Google Scholar] [CrossRef] [PubMed]
- Ronshaugen, M.; McGinnis, N.; McGinnis, W. Hox protein mutation and macroevolution of the insect body plan. Nature 2002, 415, 914–917. [Google Scholar] [CrossRef]
- Ikmi, A.; Netter, S.; Coen, D. Prepatterning the Drosophila notum: the three genes of the iroquois complex play intrinsically distinct roles. Dev. Biol. 2008, 317, 634–648. [Google Scholar] [CrossRef]
- Calleja, M.; et al. Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development 2000, 127, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- Diez del Corral, R.; Aroca, P.; Gomez-Skarmeta, J.L.; Cavodeassi, F.; Modolell, J. The Iroquois homeodomain proteins are required to specify body wall identity in Drosophila. Genes Dev. 1999, 13, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Cavodeassi, F.; Diez del Corral, R.; Campuzano, S.; Domínguez, M. Compartments and organising boundaries in the Drosophila eye: the role of the homeodomain Iroquois proteins. Development 1999, 126, 4933–4942. [Google Scholar] [CrossRef] [PubMed]
- Abzhanov, A.; Kaufman, T.C. Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev. Biol. 2000, 227, 673–689. [Google Scholar] [CrossRef]
- Choi, H.M.T.; et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 2018, 145, dev165753–122. [Google Scholar] [CrossRef] [PubMed]
- Bruce, H.S. et al. Hybridization Chain Reaction (HCR) In Situ Protocol v1. protocols.io Hybridization Chain Reaction (HCR) In Situ Protocol v1. 2021. [CrossRef]
- Kobayashi, Y. et al. Paranotal Lobes are Appendicular in Origin: Elucidation by Micro-CT Analysis of the Thoracic Muscular System in the Larvae of Carabus insulicola (Insecta, Coleoptera). Proceedings of the Arthropodan Embryological Society of Japan (2022).
- Boxshall, G.A. The evolution of arthropod limbs. Biol. Rev. 2004, 79, 253–300. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Jürgens, G. Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989, 8, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Simcox, A.A.; Cohen, S.M. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 1993, 117, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Tomlinson, A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development 1998, 125, 4483–4493. [Google Scholar] [CrossRef]
- Beermann, A.; et al. The Short antennae gene of Tribolium is required for limb development and encodes the orthologue of the Drosophila Distal-less protein. Development 2001, 128, 287–297. [Google Scholar] [CrossRef]
- Angelini, D.R.; Kaufman, T.C. Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev. Biol. 2004, 271, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Schaeper, N.D.; Prpic, N.-M.; Wimmer, E.A. A clustered set of three Sp-family genes is ancestral in the Metazoa: evidence from sequence analysis, protein domain structure, developmental expression patterns and chromosomal location. BMC Evol. Biol. 2010, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Estella, C.; Rieckhof, G.; Calleja, M.; Morata, G. The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development 2003, 130, 5929–5941. [Google Scholar] [CrossRef] [PubMed]
- Estella, C.; Mann, R.S. Non-Redundant Selector and Growth-Promoting Functions of Two Sister Genes, buttonhead and Sp1, in Drosophila Leg Development. PLoS Genet. 2010, 6, e1001001. [Google Scholar] [CrossRef] [PubMed]
- Klann, M.; Schacht, M.I.; Benton, M.A.; Stollewerk, A. Functional analysis of sense organ specification in the Tribolium castaneum larva reveals divergent mechanisms in insects. BMC Biol. 2021, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Franch-Marro, X.; Martín, N.; Averof, M.; Casanova, J. Association of tracheal placodes with leg primordia in Drosophila and implications for the origin of insect tracheal systems. Development 2006, 133, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Tomlinson, A. Initiation of the proximodistal axis in insect legs. Development 1995. [Google Scholar] [CrossRef] [PubMed]
- Letizia, A.; Barrio, R.; Campuzano, S. Antagonistic and cooperative actions of the EGFR and Dpp pathways on the iroquois genes regulate Drosophila mesothorax specification and patterning. Development 2007, 134, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Chavez, C.; Andrew, D.J. Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis. Dev. Biol. 2011, 360, 160–172. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Morphology and mechanism of the insect thorax. vol. 80 (City of Washington, Smithsonian Institution, 1927).
- Hanna, L.; Popadić, A. A hemipteran insect reveals new genetic mechanisms and evolutionary insights into tracheal system development. PNAS 2020, 121, 201908975. [Google Scholar] [CrossRef]
- Burns, K.A.; Gutzwiller, L.M.; Tomoyasu, Y.; Gebelein, B. Oenocyte development in the red flour beetle Tribolium castaneum. Dev. Genes Evol. 2012, 222, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.M.; Tomoyasu, Y. Dual evolutionary origin of insect wings supported by an investigation of the abdominal wing serial homologs in Tribolium. 2018; 115, E658–E667. [Google Scholar]
- Hu, Y.; Moczek, A.P. Wing serial homologues and the diversification of insect outgrowths: insights from the pupae of scarab beetles. Proc. R. Soc. B. 2021, 288, 20202828. [Google Scholar] [CrossRef] [PubMed]
- Tworzydlo, W.; Jaglarz, M.K.; Pardyak, L.; Bilinska, B.; Bilinski, S.M. Evolutionary origin and functioning of pregenital abdominal outgrowths in a viviparous insect, Arixenia esau. Sci. Rep. 2019, 9, 16090. [Google Scholar] [CrossRef] [PubMed]
- Hoch, H. et al. Non-sexual abdominal appendages in adult insects challenge a 300 million year old bauplan. Curr. Biol. 24, R16-7 (2014).
- Komatsu, S.; Kobayashi, Y. Embryonic development of a whirligig beetle, Dineutus mellyi, with special reference to external morphology (insecta: Coleoptera, Gyrinidae). J. Morphol. 2012, 273, 541–560. [Google Scholar] [CrossRef]
- Warren, R.W.; Nagy, L.; Selegue, J.; Gates, J.; Carroll, S. Evolution of homeotic gene regulation and function in flies and butterflies. Nature 1994, 372, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.B.; Wagner, G.P. Novelty in evolution: restructuring the concept. Annu. Rev. Ecol. Syst. 1991, 22, 229–56. [Google Scholar] [CrossRef]
- Sadier, A.; Sears, K.E.; Womack, M. Unraveling the heritage of lost traits. J Exp Zool (Mol Dev Evol) jez.b.23030 (2021). [CrossRef]
- Fritsch, M.; Richter, S. How body patterning might have worked in the evolution of arthropods—A case study of the mystacocarid Derocheilocaris remanei (Crustacea, Oligostraca). J. Exp. Zool. Pt. B 2022, 338, 342–359. [Google Scholar] [CrossRef] [PubMed]
- McCarthy-Taylor, J.B.; et al. Expression of Abdominal-B in the brine shrimp, Artemia franciscana, expands our evolutionary understanding of the crustacean abdomen. Dev. Biol. 2022, 489, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y. What crustaceans can tell us about the evolution of insect wings and other morphologically novel structures. Curr. Opin. Genet. Dev. 2021, 69, 48–55. [Google Scholar] [CrossRef]
- Grillo, M.; Casanova, J.; Averof, M. Development: A Deep Breath for Endocrine Organ Evolution. Curr. Biol. 2014, 24, R38–R40. [Google Scholar] [CrossRef]
- Fisher, C.R.; Kratovil, J.D.; Angelini, D.R.; Jockusch, E.L. Out from under the wing: reconceptualizing the insect wing gene regulatory network as a versatile, general module for body-wall lobes in arthropods. Proc. R. Soc. B 2021, 288, 20211808. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Higueras, C.; Sotillos, S.; Castelli-Gair Hombría, J. Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr. Biol. 2014, 24, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, P.; Wolff, C. External morphology of limb development in the amphipod Orchestia cavimana (Crustacea, Malacostraca, Peracarida). Zoomorphology 2005, 124, 89–99. [Google Scholar] [CrossRef]
- Hong, S.Y. Development of epipods and gills in some pagurids and brachyurans. J. Nat. Hist. 1988, 22, 1005–1040. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Jaume, D. Exopodites, epipodites and gills in Crustaceans. Arthropod Syst. Phylogeny 2009, 67, 229–254. [Google Scholar]
- Hu, Y.; Linz, D.M.; Moczek, A.P. Beetle horns evolved from wing serial homologs. Science 2019, 366, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Ohde, T.; Clark-Hachtel, C. What serial homologs can tell us about the origin of insect wings. F1000Res 2017, 6, 268–11. [Google Scholar] [CrossRef] [PubMed]
- Clark-Hachtel, C.M.; Tomoyasu, Y. Exploring the origin of insect wings from an evo-devo perspective. Curr. Opin. Insect. Sci. 2016, 13, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Clark-Hachtel, C.M.; Linz, D.M.; Tomoyasu, Y. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. 2013; 110, 16951–16956. [Google Scholar]
- DiFrisco, J.; Wagner, G.P.; Love, A.C. Reframing research on evolutionary novelty and co-option: Character identity mechanisms versus deep homology. Seminars in Cell & Developmental Biology S1084952122001033 (2022). [CrossRef]
- Averof, M.; Cohen, S.M. Evolutionary origin of insect wings from ancestral gills. Nature 1997, 385, 627–630. [Google Scholar] [CrossRef]
- Damen WG, M.; Saridaki, T.; Averof, M. Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Curr. Biol. 2002, 12, 1711–1716. [Google Scholar] [CrossRef]
- Fisher, C.R.; Wegrzyn, J.L.; Jockusch, E.L. Co-option of wing-patterning genes underlies the evolution of the treehopper helmet. Nat. Ecol. Evol. 2020, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Glassford, W.J.; et al. Co-option of an Ancestral Hox-Regulated Network Underlies a Recently Evolved Morphological Novelty. Dev. Cell 2015, 34, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Estrada, B.; Sánchez-Herrero, E. Abdominal-B antagonizes leg development. Development 2001, 9. [Google Scholar]
- Gorfinkiel, N.; Sánchez, L.; Guerrero, I. Drosophila terminalia as an appendage-like structure. Mech. Dev. 1999, 86, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Casares, F.; Sánchez, L.; Guerrero, I.; Sánchez-Herrero, E. The genital disc of Drosophila melanogaster. Dev. Genes Evol. 1997, 207, 216–228. [Google Scholar] [CrossRef]
- Boudinot, B.E. A general theory of genital homologies for the Hexapoda (Pancrustacea) derived from skeletomuscular correspondences, with emphasis on the Endopterygota. Arthropod Struct. Dev. 2018, 47, 563–613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




