Submitted:
25 January 2023
Posted:
26 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viral Samples
2.3. Infection of Hepatic Human Cells
2.4. RNA Extraction
2.5. Quantification of Viral Load by RT-qPCR
2.6. Quantification of miRNA Levels by RT-qPCR
2.7. NS1 Quantification
2.8. Apoptosis Detection
2.9. NS1 Fluorescent Imaging
2.10. Statistical Analysis
3. Results
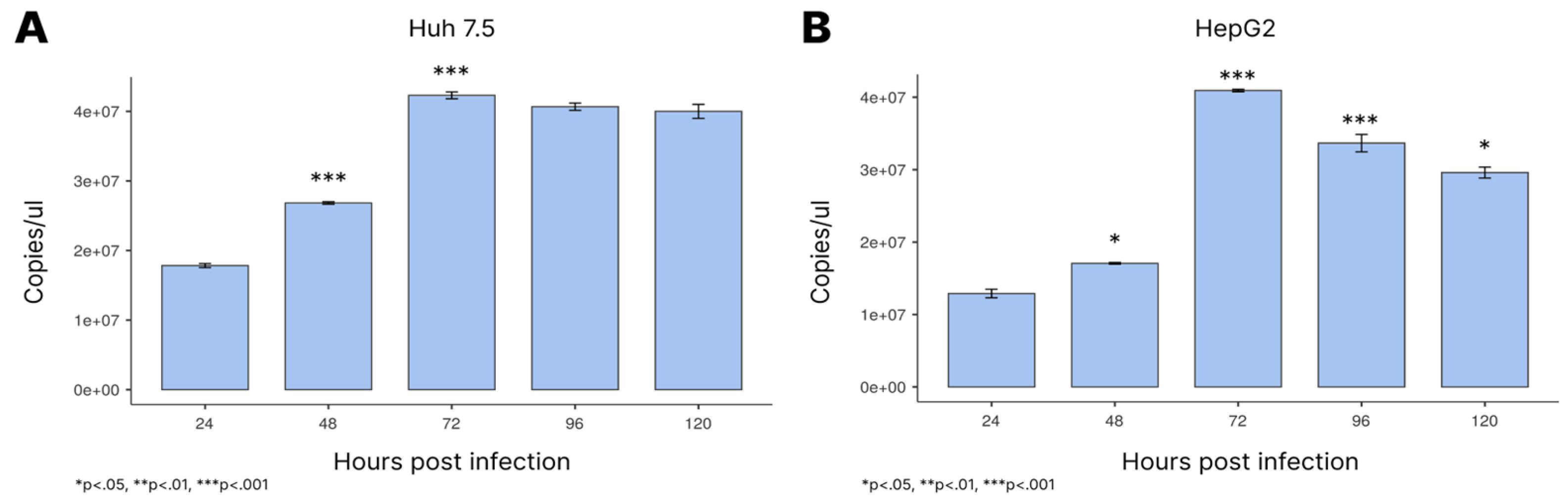
3.1. Viral Load Profile During Infection
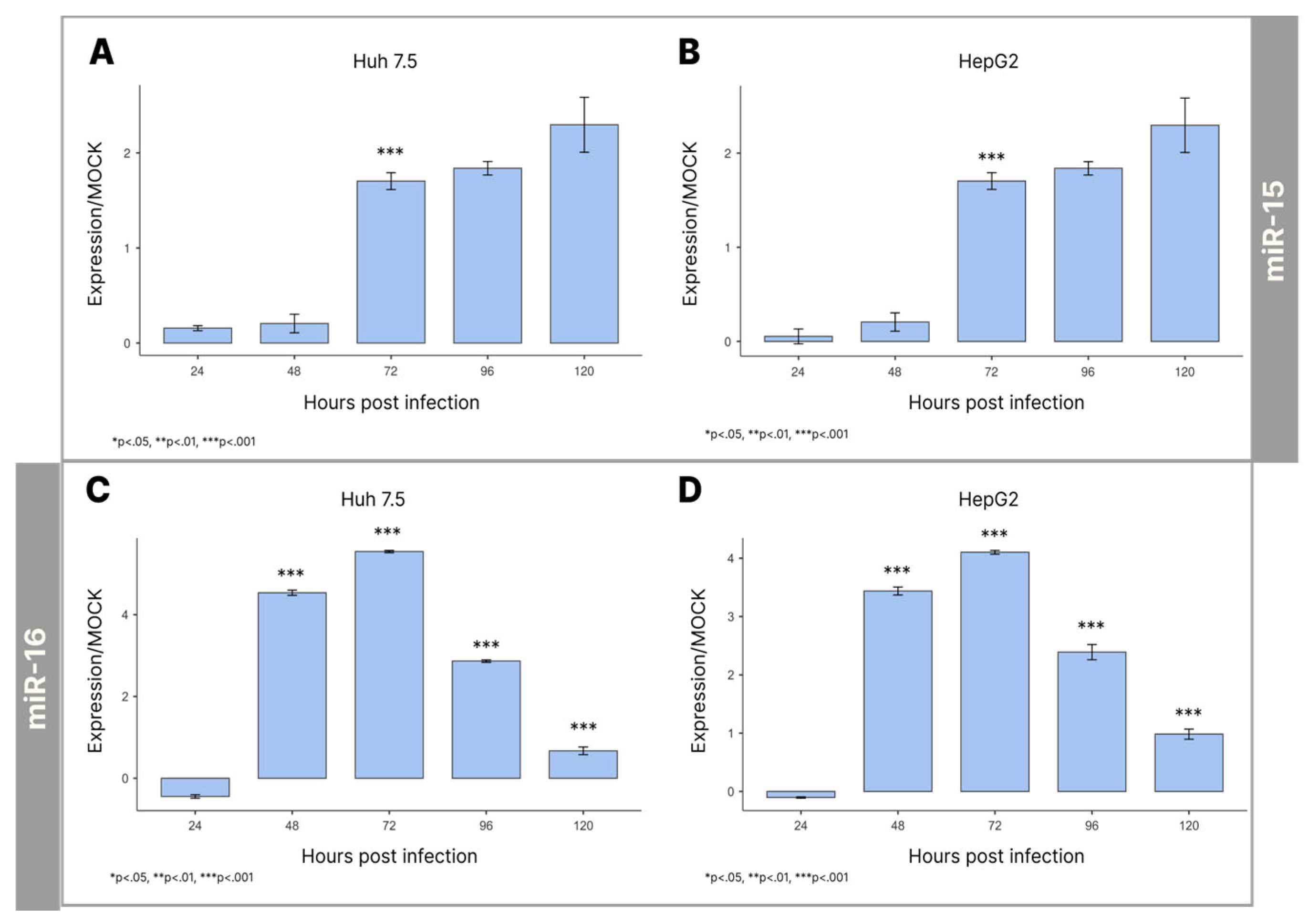
3.2. Expression Levels of miRNAs-15/16 During Infection
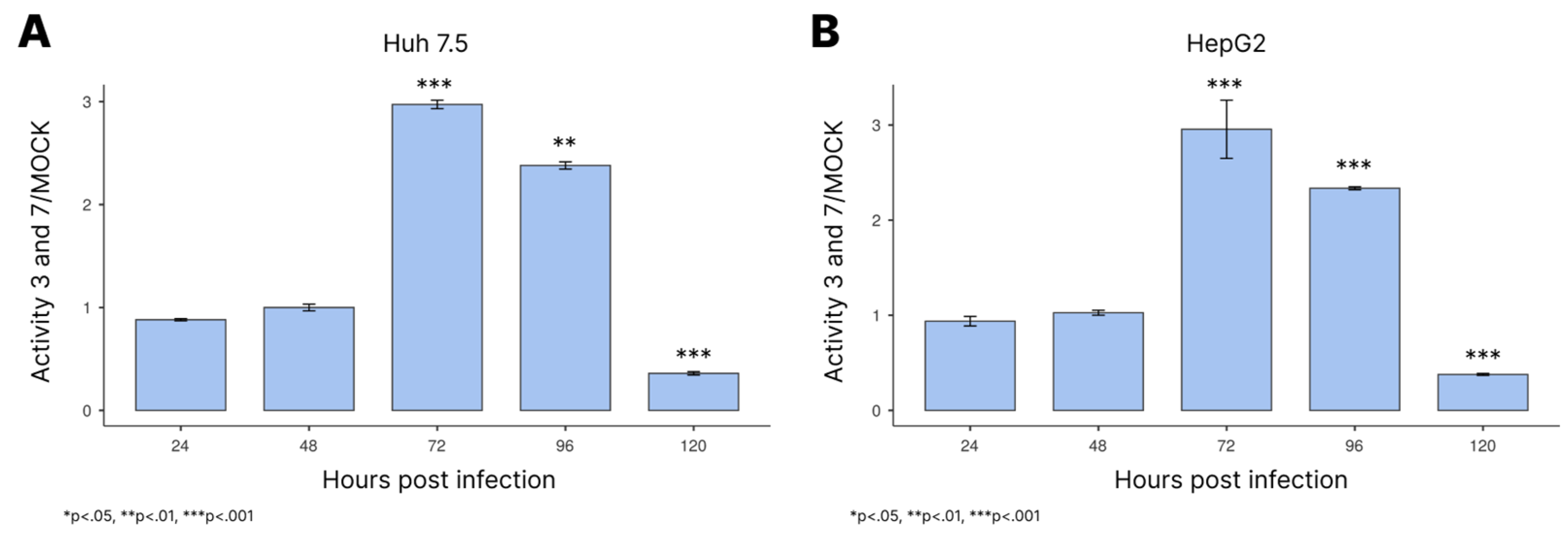
3.3. Activation of Caspases-3/7 During Infection
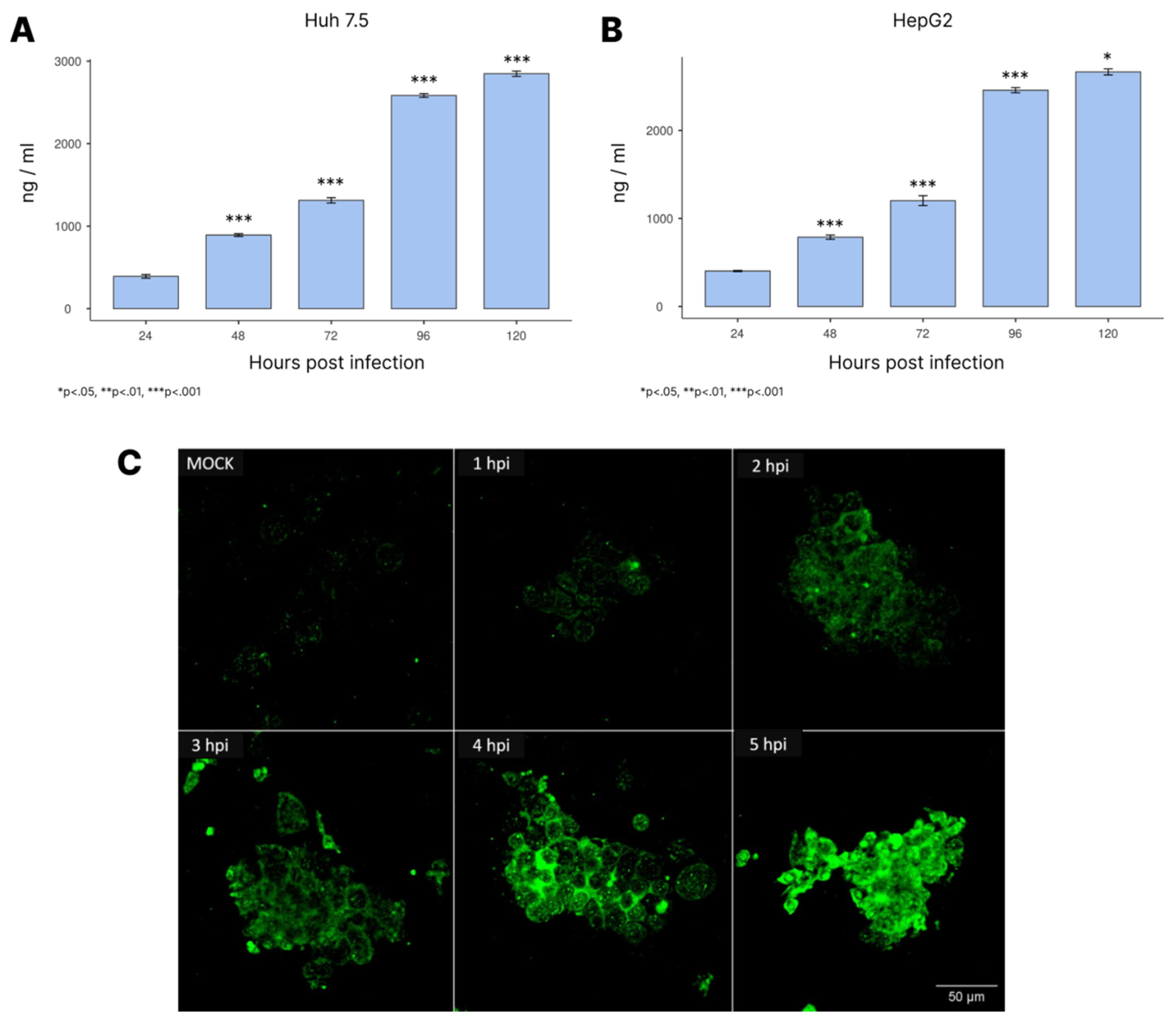
3.4. NS1 Expression During Infection
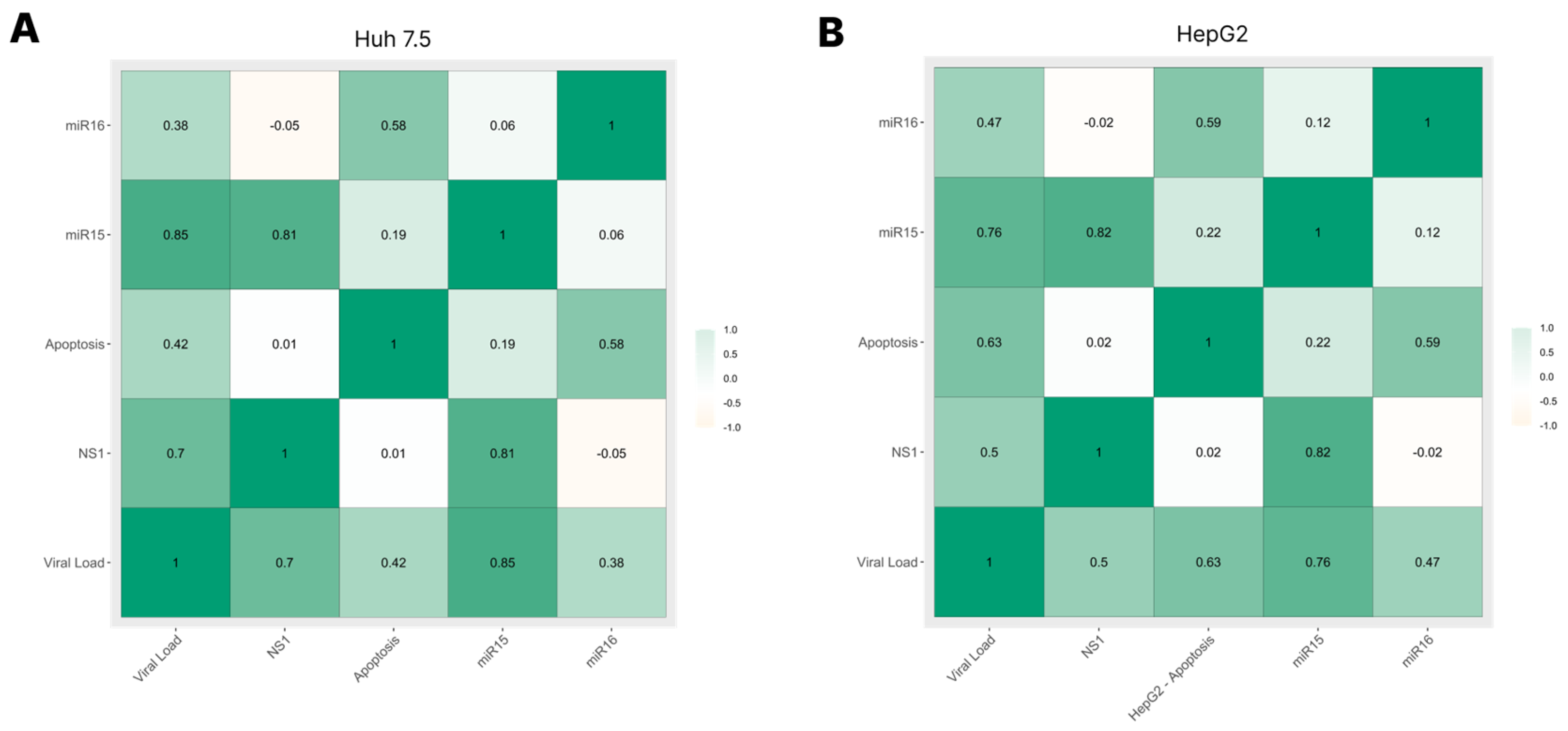
3.5. Correlation among Viral Load, NS1 Levels, miRNA-15/16 Expression, and Caspase-3/7 Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Horstick, O.; Tozan, Y.; Wilder-Smith, A. Reviewing dengue: still a neglected tropical disease? PLoS Negl. Trop. Dis. 2015, 9, e0003632. [Google Scholar] [CrossRef]
- Li, G.; Khandekar, A.; Yin, T.; Hicks, S.C.; Guo, Q.; Takahashi, K.; Lipovsky, C.E.; Brumback, B.D.; Rao, P.K.; Weinheimer, C.J.; Rentschler, S.L. Differential Wnt-mediated programming and arrhythmogenesis in right versus left ventricles. J. Mol. Cell. Cardiol. 2018, 123, 92–107. [Google Scholar] [CrossRef]
- Siqueira, J.B.; Martelli, C.M.T.; Coelho, G.E.; Simplicio, A.C. da R.; Hatch, D.L. Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerging Infect. Dis. 2005, 11, 48–53. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Dos Santos, F.B.; Barbosa, L.S.; Souza, T.M.A.; Badolato-Corrêa, J.; Sánchez-Arcila, J.C.; Nunes, P.C.G.; de-Oliveira-Pinto, L.M.; de Filippis, A.M.; Dal Fabbro, M.; Hoscher Romanholi, I.; Venancio da Cunha, R. Clinical and Laboratory Profile of Zika and Dengue Infected Patients: Lessons Learned From the Co-circulation of Dengue, Zika and Chikungunya in Brazil. PLoS Curr. Influenza 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Díaz, D.A.; Gutiérrez-Díaz, A.A.; Orozco-García, E.; Puerta-González, A.; Bermúdez-Santana, C.I.; Gallego-Gómez, J.C. Dengue virus potentially promotes migratory responses on endothelial cells by enhancing pro-migratory soluble factors and miRNAs. Virus Res. 2019, 259, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hafirassou, M.L.; Meertens, L.; Umaña-Diaz, C.; Labeau, A.; Dejarnac, O.; Bonnet-Madin, L.; Kümmerer, B.M.; Delaugerre, C.; Roingeard, P.; Vidalain, P.-O.; Amara, A. A global interactome map of the dengue virus NS1 identifies virus restriction and dependency host factors. Cell Rep. 2017, 21, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.-S.; Chen, C.-H.; Sreenu, V.; Kohl, A.; Failloux, A.-B. Assessing the potential interactions between cellular miRNA and arboviral genomic RNA in the yellow fever mosquito, Aedes aegypti. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S. Recent advances in understanding dengue. [version 1; peer review: 2 approved]. F1000Res. 2019, 8. [Google Scholar] [CrossRef]
- Halstead, S.B.; Russell, P.K.; Brandt, W.E. NS1, Dengue’s Dagger. J. Infect. Dis. 2020, 221, 857–860. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microrna biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: a review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Dou, Y.; Chen, L.; Wang, J.; Jiang, N.; Guo, C.; Yao, Q.; Wang, C.; Liu, L.; Yu, B.; Zheng, B.; Chekanova, J.A.; Ma, J.; Ren, G. Degradation of unmethylated miRNA/miRNA*s by a DEDDy-type 3’ to 5’ exoribonuclease Atrimmer 2 in Arabidopsis. Proc Natl Acad Sci USA 2018, 115, E6659–E6667. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Casseb, S.M.M.; Simith, D.B.; Melo, K.F.L.; Mendonça, M.H.; Santos, A.C.M.; Carvalho, V.L.; Cruz, A.C.R.; Vasconcelos, P.F.C. Drosha, DGCR8, and Dicer mRNAs are down-regulated in human cells infected with dengue virus 4, and play a role in viral pathogenesis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Castillo, J.A.; Urcuqui-Inchima, S. Letter to the Editor “Drosha, DGCR8, and Dicer mRNAs are downregulated in human cells infected with dengue virus 4” - Genet. Mol. Res. 15 (2): gmr.15027891 - Drosha, Dicer, and TRBP mRNA are downregulated in Vero cells with the 3’UTR of Dengue virus. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Holanda, G.M.; Casseb, S.M.M.; Mello, K.F.L.; Vasconcelos, P.F.C.; Cruz, A.C.R. Yellow Fever Virus Modulates the Expression of Key Proteins Related to the microRNA Pathway in the Human Hepatocarcinoma Cell Line HepG2. Viral Immunol. 2017, 30, 336–341. [Google Scholar] [CrossRef]
- Murphy Schafer, A.R.; Smith, J.L.; Pryke, K.M.; DeFilippis, V.R.; Hirsch, A.J. The E3 ubiquitin ligase SIAH1 targets myd88 for proteasomal degradation during dengue virus infection. Front. Microbiol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.D.; Kageyama, R.; Shehata, H.M.; Fassett, M.S.; Mar, D.J.; Wigton, E.J.; Johansson, K.; Litterman, A.J.; Odorizzi, P.; Simeonov, D.; Laidlaw, B.J.; Panduro, M.; Patel, S.; Jeker, L.T.; Feeney, M.E.; McManus, M.T.; Marson, A.; Matloubian, M.; Sanjabi, S.; Ansel, K.M. miR-15/16 Restrain Memory T Cell Differentiation, Cell Cycle, and Survival. Cell Rep. 2019, 28, 2169–2181.e4. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2019, 10, 3079. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Castillo-Medina, L.F.; Rodríguez, Y.; Pacheco, Y.; Halstead, S.; Willison, H.J.; Anaya, J.-M.; Ramírez-Santana, C. Autoimmune neurological conditions associated with zika virus infection. Front. Mol. Neurosci. 2018, 11, 116. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Alhoot, M.A.; Wang, S.M.; Sekaran, S.D. RNA interference mediated inhibition of dengue virus multiplication and entry in HepG2 cells. PLoS ONE 2012, 7, e34060. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Calin, G.A.; Croce, C.M. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010, 17, 215–220. [Google Scholar] [CrossRef]
- de Oliveira, L.F.; de Andrade, A.A.S.; Pagliari, C.; de Carvalho, L.V.; Silveira, T.S.; Cardoso, J.F.; Silva, A.L.T.E.; de Vasconcelos, J.M.; Moreira-Nunes, C.A.; Burbano, R.M.R.; Nunes, M.R.T.; Dos Santos, E.J.M.; Júnior, J.L. da S.G.V. Differential expression analysis and profiling of hepatic miRNA and isomiRNA in dengue hemorrhagic fever. Sci. Rep. 2021, 11, 5554. [Google Scholar] [CrossRef]
- Chen, C.-L.; Lin, C.-F.; Wan, S.-W.; Wei, L.-S.; Chen, M.-C.; Yeh, T.-M.; Liu, H.-S.; Anderson, R.; Lin, Y.-S. Anti-dengue virus nonstructural protein 1 antibodies cause NO-mediated endothelial cell apoptosis via ceramide-regulated glycogen synthase kinase-3β and NF-κB activation. J. Immunol. 2013, 191, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Lin, S.-C.; Chen, W.-Y.; Yen, Y.-T.; Lai, C.-W.; Tao, M.-H.; Lin, Y.-L.; Miaw, S.-C.; Wu-Hsieh, B.A. Dengue viral protease interaction with NF-κB inhibitor α/β results in endothelial cell apoptosis and hemorrhage development. J. Immunol. 2014, 193, 1258–1267. [Google Scholar] [CrossRef]
- Ouyang, X.; Jiang, X.; Gu, D.; Zhang, Y.; Kong, S.K.; Jiang, C.; Xie, W. Dysregulated Serum MiRNA Profile and Promising Biomarkers in Dengue-infected Patients. Int. J. Med. Sci. 2016, 13, 195–205. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Tsukiyama-Kohara, K. Mammalian animal models for dengue virus infection: a recent overview. Arch. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nanaware, N.; Banerjee, A.; Mullick Bagchi, S.; Bagchi, P.; Mukherjee, A. Dengue virus infection: A tale of viral exploitations and host responses. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Wong, R.R.; Abd-Aziz, N.; Affendi, S.; Poh, C.L. Role of microRNAs in antiviral responses to dengue infection. J. Biomed. Sci. 2020, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Schelter, J.; Burchard, J.; Kibukawa, M.; Martin, M.M.; Bartz, S.R.; Johnson, J.M.; Cummins, J.M.; Raymond, C.K.; Dai, H.; Chau, N.; Cleary, M.; Jackson, A.L.; Carleton, M.; Lim, L. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol. Cell. Biol. 2007, 27, 2240–2252. [Google Scholar] [CrossRef]
- El-Abd, N.E.; Fawzy, N.A.; El-Sheikh, S.M.; Soliman, M.E. Circulating miRNA-122, miRNA-199a, and miRNA-16 as Biomarkers for Early Detection of Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. Mol. Diagn. Ther. 2015, 19, 213–220. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; Rassenti, L.; Alder, H.; Volinia, S.; Liu, C.-G.; Kipps, T.J.; Negrini, M.; Croce, C.M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Pagliari, C.; Quaresma, J.A.S.; Fernandes, E.R.; Stegun, F.W.; Brasil, R.A.; de Andrade, H.F.; Barros, V.; Vasconcelos, P.F.C.; Duarte, M.I.S. Immunopathogenesis of dengue hemorrhagic fever: contribution to the study of human liver lesions. J. Med. Virol. 2014, 86, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Gyeltshen, S.; Chaijaroenkul, W.; Na-Bangchang, K. Significance of autophagy in dengue virus infection: A brief review. Am. J. Trop. Med. Hyg. 2019, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-Y.; Cheng, M.-H.; Yu, C.-Y.; Lin, Y.-S.; Yeh, T.-M.; Chen, C.-L.; Chen, C.-C.; Wan, S.-W.; Chang, C.-P. Dengue Nonstructural Protein 1 Maintains Autophagy through Retarding Caspase-Mediated Cleavage of Beclin-1. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Arboleda, J.F.; Fernandez, G.J.; Urcuqui-Inchima, S. Vitamin D-mediated attenuation of miR-155 in human macrophages infected with dengue virus: Implications for the cytokine response. Infect. Genet. Evol. 2019, 69, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Sousa, L.P.; Gomes-Ruiz, A.C.; Leite, F.G.G.; Teixeira, M.M.; da Fonseca, F.G.; Pimenta, P.F.P.; Ferreira, P.C.P.; Kroon, E.G.; Bonjardim, C.A. The dengue virus nonstructural protein 1 (NS1) increases NF-κB transcriptional activity in HepG2 cells. Arch. Virol. 2011, 156, 1275–1279. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




